Biliary intervention can be performed with conscious sedation, deep sedation, or general anesthesia. Patients should be made NPO with clear liquids only 6 hours prior to the procedure. Patients should have intravenous access and be hydrated, as manipulation of the biliary tree can cause a patient to become septic and hypotensive, requiring rapid resuscitation. Per Society of Interventional Radiology (SIR) guidelines, prophylactic antibiotics should be administered immediately prior to instrumenting the obstructed biliary tree. If culture data are available, antibiotic choice should be tailored to specific organisms. More recently, and in patients with previous biliary instrumentation, the incidence of resistant organism colonization in the bile is very high.
For a left biliary duct puncture, a subxiphoid approach is used. For a right puncture, a lateral approach is used with the cranial/caudal position as low as possible. Ideally, this will be below the 10th rib to avoid a transpleural puncture. The access needle is then passed through the hepatic parenchyma under fluoroscopic guidance. Contrast is injected through the needle as the needle is slowly retracted until a bile duct is opacified. Once a bile duct is identified, a cholangiogram is then performed using gentle short injections of contrast until adequate filling of the ducts is obtained. (It is not always possible to opacify central ducts from the initial access. Instead, a “regional” cholangiogram is performed to assess the local ducts and the access site.) If the initial access is acceptable—that is, the duct is entered as peripherally as possible and the central angles are favorable for placement of a catheter—it may be used for placement of a 6- to 8-Fr sheath. Alternatively, a second access may be obtained by choosing an acceptable peripheral duct, which has been opacified from the original injection. It may be necessary to intermittently inject contrast though the initial access to keep the ducts adequately opacified. A flexible guidewire is then advanced through the access needle into a central duct. The needle is removed and replaced with a 6- to 8-Fr sheath over the guidewire. A complete cholangiogram can then be performed through this sheath or through a 4- to 5-Fr catheter, if the sheath tip is not central enough to obtain adequate visualization of the central ducts. The obstruction is then crossed with a combination of a hydrophilic wire and 4- to 5-Fr catheter. The same wire/catheter combination is then advanced into the proximal jejunum. The hydrophilic wire is then exchanged for a nonhydrophilic stiff wire, the tract is then dilated to the appropriate size, and an internal/external biliary stent is then placed with the tip positioned as distal in the bowel as possible while maintaining the peripheral catheter side hole at the ductal site entry point. This position ensures that the ducts peripheral to the puncture site are not excluded from drainage. If the obstruction cannot be crossed, an external biliary drain is placed with its tip as central as possible. The catheter is then sutured to the skin and placed to an external gravity drainage bag. The internal/external stent can usually be capped the following day and may remain capped as long the patient is afebrile and without other signs of cholangitis.
Malignant strictures can be treated as above, or an indwelling stent may be placed in cases of palliation. The lesion is crossed using conventional techniques. Occasionally, cholangioplasty may be performed to facilitate crossing of the lesion with the stent. Self-expanding bare metal or covered stents may be utilized. Once the stent is in place, a repeat cholangiogram is performed to ensure free flow of contrast across the stent. When this has been confirmed, the parenchymal access tract is coil embolized.
Technical Tips
1.Puncture the biliary system as peripheral as possible to avoid ductal exclusion and injury of larger central vessels. (Fig. 34.1 shows large intrahepatic hematoma from laceration of hepatic artery.)
2.During pullback injection, small “puff” of contrast should be introduced. Contrast in the portal vein and hepatic artery will head to the periphery of the liver and clear completely. The hepatic vein will clear centrally toward the heart.
3.Overinjecting a dilated infected biliary system can lead to sepsis, shock, and death.
4.If the wire does not initially advance smoothly, the entry angle of the duct may be too acute or the needle may be just outside the duct. Pulling back and repeating a more peripheral puncture will likely be helpful.
5.A short nitinol wire, increased fluoro magnification, and twirling movements of the wire may be beneficial.
6.Advancing a 6-Fr 25-cm-long sheath immediately proximal to the obstruction will allow more forward force to be placed on the coaxial catheters and guidewires.
7.The duct distal to the obstruction can be punctured, and a snare can be placed allowing through and through access.
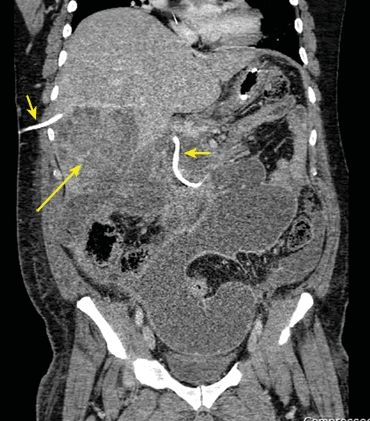
FIGURE 34.1 Coronal CT showing large intrahepatic hematoma (long arrow) from inadvertent hepatic artery laceration during percutaneous transhepatic cholangiography (PTC) (short arrows) placement.
Postprocedural Management
The patient should have his or her vital signs checked frequently immediately after the procedure. While procedure-related complications following biliary catheter placement are infrequent, bleeding from injury to the hepatic artery or portal vein is possible. This usually manifests as bleeding through or around the catheter, but would also be suggested by tachycardia with or without hypotension. Cholangiography might demonstrate communication between the catheter tract and blood vessel. Portal bleeding is usually easily treated by replacement of the indwelling catheter with a bigger catheter and/or one advanced more centrally such that its side holes no longer communicate with the portal vein. Hepatic arterial bleeding is usually treated with embolization of the injured artery. Pneumothorax and the equally uncommon bilious pleural effusion are rare complications; both are treated by catheter drainage of the pleural space.
The external or internal/external biliary catheter should undergo routine catheter care including daily site cleaning and dressing changes. Biliary catheters, whether external drains or internal/external catheters, should be flushed: Our standard procedure is to flush catheters with 10 mL of sterile saline twice a day. Bile leak around the catheter, persistent pericatheter or right upper quadrant pain, and/or difficulty flushing the catheter are all indications for cholangiography and possible catheter replacement.
Patients are observed overnight for symptomatic management and to allow for early detection of delayed procedural complications. At our institution, patients with benign bile duct strictures are normally managed with 12 months of stenting. Once the initial stent has been placed (usually 10 to 12 Fr), it is serially upsized every few months to 20 Fr as tolerated by the patient. The 12-month timeframe starts once the 20-Fr stent has been placed. The stent is exchanged every 3 months during this time. At 12 months, serum liver chemistry tests and cholangiogram are performed. If contrast flows freely through the treated stricture, a “clinical trial” is initiated. This involves placing a new catheter such that the tip is proximal to the previous stricture. The catheter is then capped for 2 weeks, at which time the patient returns for a repeat cholangiogram and serum liver chemistry evaluation. If the liver chemistry tests are stable, the cholangiogram shows resolved stricture, and the patient is doing well clinically (e.g., no fevers or signs of cholangitis), the catheter is removed. An alternative to the clinical trial to test the integrity of a treated bile duct stricture is the Whittaker test, which involves manometry measurement of bile duct pressure proximal and distal to the stricture. Patients with “definitively” treated benign bile duct strictures must have long-term annual follow-up of liver chemistry tests with their primary physician, gastroenterologist, or HPB surgeon.
Immediate Complications
1.Bleeding (subcapsular/intraparenchymal/peritoneal/pleural/biliary—hemobilia)
2.Infection (cholangitis/sepsis)
3.Bile duct perforation
4.Stent migration/malposition
Delayed Complications
1.Infection (pancreatitis/cholecystitis/cholangitis)
2.Pancreatitis
3.Bile leak in the peritoneum or pleural space
4.Stent occlusion, migration, kinking, or dislodgement
5.Biloma
The average rate of major complications is about 2%, although complication rates can vary from 4% to 25% depending on the institution and operator experience. The majority of these complications can be treated by the interventionalist. Pleural transgression is usually treated with a chest tube. A patient who is bleeding should be treated with standard resuscitative measures. In addition, coagulopathy secondary to jaundice must be corrected (administration of vitamin K and replacing plasma clotting factors). Bleeding in and around a catheter is usually due to a side hole within a blood vessel that was crossed. Advancing the catheter will stop the majority of the bleeding that is venous. If the patient continues to bleed, an arteriogram should be performed with the biliary catheter removed over a wire, maintaining access. Coil embolization distal and proximal to the bleeding arterial site is therapeutic. Infectious cholangitis should be treated with 7 to 10 days of antibiotic coverage for biliary/gastrointestinal (GI) bacteria. Additionally, a cholangiogram should be performed to ensure the catheter is functioning properly. Sepsis or septic shock requires ICU admission. Insertion-site cellulitis may suggest misplaced side holes and should be managed with cholangiography with catheter replacement, if indicated. If cellulitis is present with a well-positioned patent catheter, then antibiotics should be administered. Biliary catheter obstruction usually requires reevaluation with repeat cholangiogram and replacement. Biliary catheter dislodgement should prompt urgent evaluation as newly created tracts may close within hours. Stent occlusion can be treated with recanalization and cholangioplasty, or new stent placement.
Outcomes/Follow-up
Effective biliary drainage is established in close to 100% of bile ducts accessed in appropriately selected patients. Percutaneous drainage can successfully treat between 70% and 100% of iatrogenic bile duct injuries. Reported technical success rate for nontransplant bile duct strictures approaches 100% with transplant stenosis having a more modest success rate of 45% to 80%. The covered stent patency for biliary obstruction is 90% to 95%, 76% to 92%, and 76% to 85% at 3, 6, and 12 months, respectively.
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT
A TIPS is a percutaneous technique used to treat portal hypertension and its complications. A TIPS is placed to facilitate reduction of the portal venous pressure by placing a decompressive stent between a hepatic vein and an intrahepatic portal vein (Fig. 34.2 shows TIPS). Since its inception over 20 years ago, more than 1,000 patients have been enrolled in multiple controlled trials studying efficacy and safety of the procedure. Furthermore, more than 1,000 papers have been published allowing for the development of guidelines to define the role of TIPS in the management of portal hypertension.
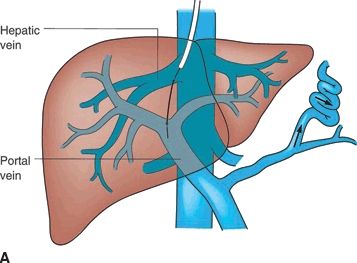
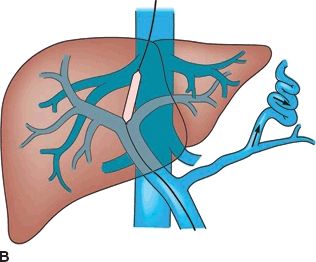
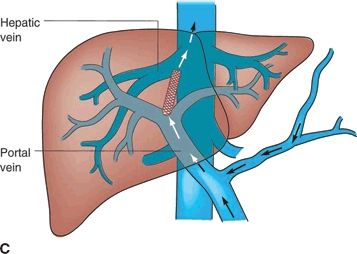
FIGURE 34.2 (A-C). Schematic of TIPS. (From Mulholland MW, Lillemoe KD, Doherty GM, et al. Greenfield’s surgery: scientific principles & practice, 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2010.)
Cirrhosis is common throughout the world and may lead to ascites formation, variceal bleeding, and hepatic encephalopathy. Of these complications, variceal bleeding is the most common indication for TIPS. In fact, the mortality rate within 6 weeks of bleeding is 30%, which can most commonly be attributed to uncontrolled or early recurrent bleeding.
The model for end-stage liver disease (MELD) score was developed specifically to predict post-TIPS outcomes. This formula, which takes into account the creatinine, bilirubin, and international normalized ratio (INR), is an accurate predictor of 30-day mortality. If the MELD score is 1 to 10, the 30-day mortality is 3.7%. If the MELD is greater than 24, the 30-day mortality rises to 60%.
As the name indicates, TIPS is placed via a right internal jugular vein approach. It may be performed with moderate sedation or general anesthesia. The type of stent that is placed is operator dependent. Current evidence suggests that a PTFE-covered stent (Viatorr) may have the best long-term outcomes.
Technical success rates are greater than 95%. Hemodynamic success rates are greater than 95%. Clinical success rates are measured in terms of survival, control of bleeding, or ascites. Control of bleeding is achieved in 90%, and improved ascites control is seen in 60% to 85%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree