Interferons
The interferons (IFNs) are a family of proteins that are grouped into three classes α, β, and γ. They were discovered based on their ability to “interfere” with viral infection of cells. Subsequent study has revealed a panoply of biological actions including immunomodulatory, antiproliferative, and antiangiogenic effects (1). Nearly all the oncologic applications of the IFNs have been of the α class.
The α and β IFNs are encoded by a series of genes on chromosome 9p. At least 12 varieties of α IFN exist. A product composed of several species of α IFN produced by stimulated lymphoblasts exists (Wellferon, Burroughs Wellcome), but the predominant forms of IFN in clinical use are recombinant molecules of a single species of α, specifically α2. IFN-α2 is 165 amino acids in length with a molecular weight of about 23 kD. IFN-α2a (Hoffmann-La Roche) differs from IFN-α2b (Schering-Plough) by a single amino acid; IFN-α2a has a lysine at position 23, and IFN-α2b has an argi-nine. IFN-β has no established role in cancer treatment but is widely used to suppress relapses in multiple sclerosis.
IFN-γ maps to chromosome 12, is 143 amino acids in length, and has minimal sequence homology with IFNs α and β. Its cellular receptor is distinct from the receptor for IFNs α and β, but both types of receptors are widely expressed on all nucleated cells and tissues. Each cell expresses 100–2000 receptors, and the binding constants (Kd) are between 10–11 and 10–9 M. The α receptor has two chains, one of which is associated with Tyk2 tyrosine kinase and one with JAK1 kinase (2). The genes for the a receptor map to chromosome 21q22.1. The γ receptor also has two chains, one of which is associated with JAK1 kinase and one with JAK2 kinase. The γ receptor genes are on chromosome 6q. Figure 13-1 shows the two forms of receptor for the three classes of IFNs.
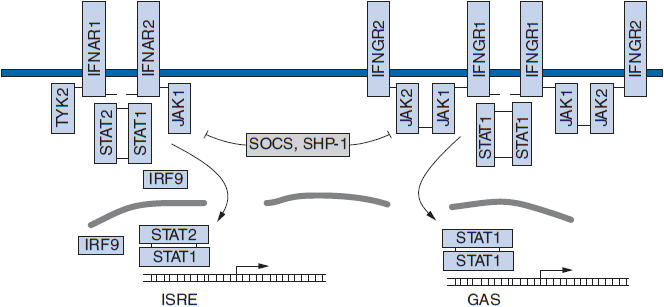
FIGURE 13-1 Components of the interferon (IFN) signaling pathways. The major components responsible for relaying IFN-mediated signals from the cell surface to the regulatory elements of IFN-stimulated genes are represented. GAS, IFN-γ activated site; IFNAR, IFN-α receptor; IFNGR, IFN-γ receptor; ISRE, IFN-stimulated response element; JAK, Janus kinase; SHP, width=”540″ height=”458″ src-homology 2 domain-containing protein tyrosine phosphatase; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; Tyk, JAK family kinase. Small black bars represent tyrosine residues that become phosphorylated and induce complex formation.
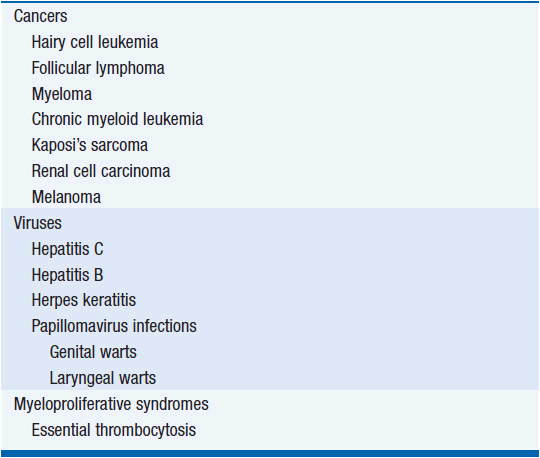
IFNs have been approved for use in seven types of cancer, several viral diseases, an autoimmune disease (multiple sclerosis; IFN-β), and an immune deficiency disease (chronic granulomatous disease; IFN-γ) (Table 13-1). In addition to the tumors listed in Table 13-1, IFN-α also has antitumor activity in cutaneous T-cell lymphoma. However, for most of these cancers, IFN is a second- or third-line alternative.
MECHANISM OF ACTION
The wide range of biologic effects of the IFNs has made it difficult to determine a single central mechanism of action. The fact that responses appear to correlate roughly with dose suggests that direct antitumor mechanisms predominate. IFN-α and IFN-β may exert direct antitumor effects and are capable of boosting mainly innate host defenses. IFN-γ appears to have minimal direct effects on tumor cells but is a potent mediator of effects on immune cells. As a cytokine produced by CD4+ Th1 cells, IFN-γ promotes cytolytic activity from CD8+ cytotoxic T cells. Cells exposed to IFNs are induced to express literally hundreds of new gene products (see http://www.lerner.ccf.org/labs/williams/der.html). They induce cyclin-dependent kinase inhibitors to cause cell cycle arrest and induce FAS and caspases, components of apoptosis pathways (3). IFNs also induce alterations in host defenses. They increase CD8+ cytotoxic T-cell activity, increase NK activity, and stimulate macrophages and dendritic cells. They induce an upregulation of class I MHC molecules in tumors, which could result in more effective recognition of target cells by cytotoxic T cells. In addition, IFNs induce the expression of some known tumor-associated antigens. Many cell effects of IFNs are mediated through the action of a family of proteins called IFN regulatory factors (IRFs) (4). IFNs also inhibit the expression of basic fibroblast growth factor and vascular endothelial growth factor, cytokines involved in tumor angiogenesis.
The in vivo mechanisms of action have not been defined. When biological effects of IFN are measured in man, the assays usually test levels of neopterin (produced by IFN-stimulated monocytes) or [β2-microglobulin (shed by IFN-stimulated cells) in the serum or measure the induction of the IFN-inducible 2–5 oligo A synthetase in mononuclear cells.
PHARMACOLOGY
IFN-α is generally administered intramuscularly or subcutaneously. About 80% of an injected dose is absorbed. It is absorbed with a t1/2 of 2–2½ h and eliminated with a t1/2 of 3–8 h. An intramuscular dose of 72 million units usually produces peak serum levels of 300–500 U/ml (5). The intravenous administration of 20 million units/m2 produces peak serum levels of about 2500 U/ml. The maximum tolerated dose of IFN-α depends on the route of administration, the frequency of dosing, the duration of treatment, and the patient’s willingness to accept toxicities (see below). Most people can tolerate 3–5 million units 3 times a week on a continuous basis.
Efforts to alter the pharmacokinetics of the molecule have been made by attaching polymers of polyethylene glycol (PEG) to the parent molecule (6). Hoffmann-La Roche attached a 40-kD branched chain molecule of PEG to its IFN-α2a and Schering-Plough attached a 25-kD linear chain of PEG to its IFN-α2b. PEG-IFN-α2a has an absorption half-life of 50 h, an elimination half-life of 65 h, and time to maximum serum concentration of 48–80 h. The maximum tolerated dose for PEG-IFN-α2a is 450 μg per week. PEG-IFN-α2b has an absorption half-life of 4–5 h, an elimination half-life of about 40 h, and a time to maximum serum concentration of 15–44 h. The maximum tolerated dose for PEG-IFN-a2b is around 6 μg/kg per week. These pegylated forms sustain measurable blood levels of IFN over a longer period of time. Pegylation may improve the antiviral efficacy of IFN in hepatitis C treatment, but comparisons of efficacy between native and pegylated IFN preparations have been limited in cancer indications. Unpegylated and pegylated IFN appear to be comparably active in chronic myeloid leukemia (7).
TOXICITIES
IFN induces severe flu-like symptoms including fever, chills, rigors, myalgias, arthralgias, malaise, and somnolence in the initial stages of treatment (Table 13-2). If treatment continues, over time these symptoms abate as a reflection of tachyphylaxis. If the course of therapy is interrupted for even short periods, the flu-like symptoms may return upon restarting IFN treatment. With chronic administration, patients often develop severe fatigue, depression, anorexia, and weight loss. These are the major symptoms that may cause an interruption in the course of therapy. Aside from the systemic and nervous system toxicities, myelosuppression and hepatic toxicity are the major organ toxicities. Hypertriglyceridemia is common. Rare patients, particularly those with T-cell tumors, can develop nephrotic syndrome and acute renal failure. Some patients develop autoimmune disorders such as thyroiditis, and some with preexisting autoimmune disease experience an exacerbation of symptoms on IFN.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree