Insulin Secretion in Vivo
Melissa K. Cavaghan
Kenneth S. Polonsky
The classic experiments of Von Mering and Minkowski at the turn of the last century demonstrating that pancreatectomy in dogs resulted in hyperglycemia (1) focused attention on the important role of the pancreas in maintaining glucose homeostasis in vivo. Banting and Best (2), by reversing this hyperglycemia with internal secretions of the pancreas, confirmed the belief that the islets of Langerhans were the key cells within the pancreas responsible for maintaining normal blood glucose levels. Although the isolation and purification of insulin rapidly followed, many years passed before sensitive techniques for evaluating β-cell function were devised. Because these techniques are critical to the analysis of insulin secretion in vivo, they will be discussed at the outset.
METHODS OF QUANTITATING β-CELL FUNCTION
The development of a sensitive radioimmunoassay for the measurement of insulin levels was the first major advance in our attempts to understand how the β-cell functions in vivo (3). For many years afterward, the measurement of peripheral levels of insulin was the gold standard used to evaluate β-cell secretory activity (4,5,6,7,8). This approach, however, is limited by the fact that 50% to 60% of the insulin produced by the pancreas is extracted by the liver without ever reaching the systemic circulation (9,10). While these problems can, in fact, be overcome by hepatic vein catheterization allied to intraportal infusion of insulin, these techniques can only be applied in an investigational setting and, even then, are only of value under steady-state conditions (11). The standard radioimmunoassay for the measurement of insulin concentrations is also limited by its inability to distinguish between endogenous and exogenous insulin, making it ineffective as a measure of endogenous β-cell reserve in the insulin-treated diabetic patient. The problem is further compounded by the development in many of these patients of antibodies to insulin, which interfere in the interpretation of serum levels of immunoreactive insulin. Although insulin-specific assays have been developed, another disadvantage of the conventional insulin radioimmunoassay is its inability to distinguish between levels of circulating proinsulin and true levels of circulating insulin.
Following the discovery of proinsulin, the single-chain precursor of insulin (12), and the identification of the biosynthetic pathway of insulin within the β-cell (13), β-cell secretory products in addition to insulin were found in the circulation. These included proinsulin, proinsulin conversion intermediates (split proinsulins), and connecting-peptide (C-peptide) (see Chapter 5). Within the islet cells, proinsulin undergoes cleavage at the Golgi apparatus (14), a reaction that leads to the formation of insulin, C-peptide, and two pairs of basic amino acids. Insulin is subsequently released into the circulation at concentrations equimolar to those of C-peptide (13,14,15). In addition, small amounts of intact proinsulin and proinsulin conversion intermediates are released. Although these molecules constitute 20% of the total circulating
insulin-like immunoreactivity (16), they are much less potent than insulin biologically. It has been estimated that the biologic potency of proinsulin in vivo is only 10% of that of insulin (17,18), whereas the potency of split proinsulin is between that of proinsulin and insulin (19,20). The low concentrations of proinsulin and split proinsulins in serum, however, ensure that in vivo, under normal physiologic conditions, their effects are negligible. In contrast to insulin and proinsulin, C-peptide has no known conclusive effects on carbohydrate metabolism (21,22). It has recently been suggested that it may stimulate Na+/K+-ATPase and endothelial nitric oxide synthase activities, resulting in a number of biologic activities, including augmented blood flow in skeletal muscle and skin, diminished glomerular hyperfiltration, reduced urinary albumin excretion, and improved nerve function in patients with type 1 diabetes mellitus who lack C-peptide, but not in healthy subjects (23). It has therefore been proposed that replacement of C-peptide together with insulin may prevent or retard the progression of the long-term complications of diabetes mellitus (24,25). Unlike insulin, C-peptide is not extracted by the liver (10,26,27) and is excreted almost exclusively by the kidneys. Its plasma half-life of approximately 30 minutes (28) contrasts sharply with that of insulin, which is approximately 4 minutes.
insulin-like immunoreactivity (16), they are much less potent than insulin biologically. It has been estimated that the biologic potency of proinsulin in vivo is only 10% of that of insulin (17,18), whereas the potency of split proinsulin is between that of proinsulin and insulin (19,20). The low concentrations of proinsulin and split proinsulins in serum, however, ensure that in vivo, under normal physiologic conditions, their effects are negligible. In contrast to insulin and proinsulin, C-peptide has no known conclusive effects on carbohydrate metabolism (21,22). It has recently been suggested that it may stimulate Na+/K+-ATPase and endothelial nitric oxide synthase activities, resulting in a number of biologic activities, including augmented blood flow in skeletal muscle and skin, diminished glomerular hyperfiltration, reduced urinary albumin excretion, and improved nerve function in patients with type 1 diabetes mellitus who lack C-peptide, but not in healthy subjects (23). It has therefore been proposed that replacement of C-peptide together with insulin may prevent or retard the progression of the long-term complications of diabetes mellitus (24,25). Unlike insulin, C-peptide is not extracted by the liver (10,26,27) and is excreted almost exclusively by the kidneys. Its plasma half-life of approximately 30 minutes (28) contrasts sharply with that of insulin, which is approximately 4 minutes.
Because C-peptide is secreted in equimolar concentrations with insulin and is not extracted by the liver, many investigators have used levels of C-peptide as a marker of β-cell function. While C-peptide levels are usually measured in plasma, C-peptide levels in urine have also been used to evaluate endogenous insulin secretion (29,30,31,32). This approach is limited, however, because the fraction of the secreted C-peptide that appears in the urine varies considerably among subjects and even in the same subject studied on different occasions (33). The use of plasma C-peptide levels as an index of β-cell function is dependent on the critical assumption that the mean clearance rates of C-peptide are constant over the range of C-peptide levels observed under normal physiologic conditions. This assumption has been shown to be valid for both dogs and humans (10,34), and this approach can be used to derive rates of insulin secretion from plasma concentrations of C-peptide under steady-state conditions (34). However, because of the long plasma half-life of C-peptide, under non-steady-state conditions (e.g., following a glucose infusion), peripheral plasma levels of C-peptide do not change in proportion to the changing insulin secretory rate (34,35). Thus, under these conditions, insulin secretion rates are best calculated with use of the two-compartment model initially proposed by Eaton and coworkers (36). This approach involves nonlinear least-squares regression analysis of C-peptide decay curves to derive model parameters in individual subjects. Once the fractional rate constants and distribution volume are known, the peripheral concentrations of C-peptide can be analyzed mathematically and the corresponding secretion rates derived. Estimates of the secretion rate of insulin in human subjects by this method are quite accurate—reportedly 98% ± 3% of the actual rate as rates of insulin secretion are increasing and 100% ± 2% as they are decreasing (34). Similar findings have been reported for dogs (37). Modifications to the C-peptide model of insulin secretion have recently been introduced. This approach combines the minimal model of insulin action with the two-compartment model of C-peptide kinetics and allows insulin secretion and insulin sensitivity to be derived following either the intravenous or the oral administration of glucose (38,39,40,41).
Rates of insulin secretion have also been measured by calculating the difference between arterial and hepatic venous C-peptide and by multiplying this difference by the estimated hepatic plasma flow (42,43), an approach designed to overcome the inherent difficulty of performing portal venous cannulation in humans. Rates of insulin secretion determined by this method are similar to those obtained with other methods, but the technique is invasive and by its nature can only be applied in an investigational setting.
In summary, under steady-state conditions, levels of C-peptide in whole plasma provide an accurate index of the insulin secretory rate, while under non-steady-state conditions, rates of β-cell secretion of insulin can be derived more accurately and easily from mathematical analysis of peripheral C-peptide concentrations with use of a two-compartment model. In interpreting the validity of experimental results evaluating insulin secretion in vivo, one should always take into account the limitations of the method used to assess β-cell function.
REGULATION OF INSULIN SECRETION
Carbohydrate Nutrients
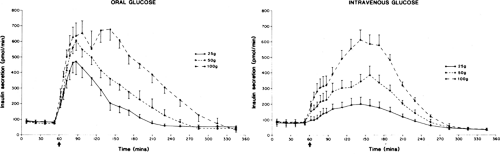
The most important physiologic substance involved in the regulation of insulin release is glucose (44,45,46). The effect of glucose on the β-cell is dose-related. Dose-dependent increases in concentrations of insulin and C-peptide and in rates of insulin secretion have been observed following oral and intravenous glucose loads, with 1.4 units (∼50 μg) of insulin, on average, being secreted in response to an oral glucose load as small as 12 g (42,47,48,49) (Fig. 7.1). The insulin secretory response is greater
after oral than intravenous glucose administration (49,50,51,52). Known as the incretin effect (48,53), this enhanced response to oral glucose has been interpreted as an indication that absorption of glucose by way of the gastrointestinal tract stimulates the release of hormones and other mechanisms that ultimately enhance the sensitivity of the β-cell to glucose (see discussion on hormonal factors below and Chapter 12). In a study involving nine normal volunteers who received a glucose infusion at a rate designed to achieve levels previously attained following an oral glucose load, the amount of insulin secreted in response to the intravenous load was 26% less than that secreted in response to the oral load (52).
after oral than intravenous glucose administration (49,50,51,52). Known as the incretin effect (48,53), this enhanced response to oral glucose has been interpreted as an indication that absorption of glucose by way of the gastrointestinal tract stimulates the release of hormones and other mechanisms that ultimately enhance the sensitivity of the β-cell to glucose (see discussion on hormonal factors below and Chapter 12). In a study involving nine normal volunteers who received a glucose infusion at a rate designed to achieve levels previously attained following an oral glucose load, the amount of insulin secreted in response to the intravenous load was 26% less than that secreted in response to the oral load (52).
Insulin secretion does not respond as a linear function of glucose concentration. The relationship of glucose concentration to the rate of insulin release follows a sigmoidal curve, with a threshold corresponding to the glucose levels normally seen under fasting conditions and with the steep portion of the dose-response curve corresponding to the range of glucose levels normally achieved postprandially (54,55,56). The sigmoidal nature of the dose-response curve has been attributed to a gaussian distribution of thresholds for stimulation among the individual β-cells (56,57,58).
When glucose is infused intravenously at a constant rate, an initial biphasic secretory response is observed that consists of a rapid, early insulin peak followed by a second, more slowly increasing, peak (44,59,60). The significance of the first-phase insulin release is unclear but may reflect the existence of a compartment of readily releasable insulin within the β-cell or a transient increase and decrease of a metabolic signal for insulin secretion (61). Despite early suggestions to the contrary (62,63), a subsequent study has demonstrated that the first-phase response to intravenous glucose is highly reproducible within subjects (64). Following the acute response, a second phase of insulin release occurs that is directly related to the level of glucose elevation. In vitro studies of isolated islet cells and the perfused pancreas have identified a third phase of insulin secretion commencing 1.5 to 3.0 hours after exposure to glucose and characterized by a spontaneous decline in secretion to 15% to 25% of the amount released during peak secretion—a level subsequently maintained for more than 48 hours (65,66,67,68).
The effects of a variety of other sugars, sugar derivatives, and sugar alcohols on the β-cell have also been examined (69). d-glucose, d-mannose, d-glyceraldehyde, dihydroxyacetone, d-glucosamine, N -acetylglucosamine, fructose, and galactose have all been shown to be stimulators or potentiators of insulin secretion in vitro. In vivo studies in dogs and humans suggest that xylitol and sorbitol also enhance β-cell function.
The insulin secretory response to glucose exhibits anomeric specificity, the α-anomer being a more potent stimulator of insulin release than the β-anomer (70). Similar results have been obtained with mannose (71). Because α-anomers are more readily metabolized by the glycolytic pathway than are β-anomers (72,73), it has been suggested that the metabolism of glucose and mannose within the β-cell is a prerequisite for the production of intracellular signals that trigger insulin release in response to these secretagogues. In support of this suggestion is the observation that mannoheptulose, an inhibitor of the glycolytic enzyme glucokinase, blocks the insulin secretory response to glucose. Similarly, iodoacetate, an inhibitor of glyceraldehyde dehydrogenase, blocks the β-cell response to hexoses (74).
Noncarbohydrate Nutrients
Amino acids have been shown to stimulate insulin release in the absence of glucose, the most potent secretagogues being the essential amino acids leucine, arginine, and lysine (75,76). The effects of arginine and lysine on the β-cell appear to be more potent than those of leucine. Although the effects of amino acids on insulin secretion are independent of concomitant changes in glucose levels, the effects are potentiated by glucose (76,77,78). The response of the islet cells to a series of amino acid metabolites has also been evaluated. Phenylpyruvate, α-ketoisocaproate, α-keto-β-methylvalerate, and α-ketocaproate are potent stimulators of insulin release, and most are effective in the absence of glucose (69,79).
In contrast to amino acids, various lipids and their metabolites appear to have only minor effects on insulin release in vivo. Although carbohydrate-rich fat meals stimulate insulin secretion, carbohydrate-free fat meals have minimal effects on β-cell function (80). Ketone bodies and short- and long-chain fatty acids have been shown to acutely stimulate insulin secretion both in isolated islet cells and in humans (81,82,83,84,85). The effects of elevated free fatty acids in the insulin secretory responses to glucose are related to the duration of the exposure. Zhou and Grill first suggested that long-term exposure of pancreatic islets to free fatty acids inhibited glucose-induced insulin secretion and biosynthesis (86). This observation has been confirmed in rats (87). In humans, it was demonstrated that the insulin resistance induced by an acute (90-minute) elevation in free fatty acids was compensated for by an appropriate increase in insulin secretion (88). Following chronic elevation of free fatty acids (48 hours), the β-cell compensatory response for insulin resistance was not adequate. Additional studies have demonstrated that the adverse effects of prolonged elevations in free fatty acids on glucose-induced insulin secretion are not seen in individuals with type 2 diabetes. On the basis of these results, it appears that elevated free fatty acids may contribute to the failure of β-cell compensation for insulin resistance.
Hormonal Factors
The release of insulin from the β-cell following a meal is facilitated by a number of gastrointestinal peptide hormones, including glucose-dependent insulinotropic peptide (GIP), cholecystokinin, and glucagon-like peptide-1 (GLP-1) (53,89,90,91,92,93,94,95,96) (see Chapter 12). These hormones are released from intestinal endocrine cells postprandially and travel in the bloodstream to reach the β-cells, where they act through second messengers to increase the sensitivity of these islet cells to glucose. In general, these hormones are not of themselves secretagogues, and their effects are only evident in the presence of hyperglycemia (89,90,91). The release of these peptides may explain why the modest postprandial glucose levels achieved in normal subjects in vivo have such a dramatic effect on insulin production whereas similar glucose concentrations in vitro elicit a much smaller response (96). Similarly, this so-called incretin effect could account for the greater β-cell response observed following oral as opposed to intravenous glucose administration. Whether impaired postprandial secretion of incretin hormones plays a role in the inadequate insulin secretory response to oral glucose and to meals in impaired glucose tolerance (IGT) or diabetes mellitus is controversial (97,98,99,100,101,102,103,104), but pharmacologic doses of these peptides may have future therapeutic benefits. Subcutaneous administration of GLP-1, the most potent of the incretin peptides, lowers glucose levels in patients with type 2 diabetes by stimulating endogenous insulin secretion and perhaps by inhibiting glucagon secretion and gastric emptying (105,106). Because of the short half-life of GLP-1, however, its longer-acting analogue, exendin-4, has greater therapeutic promise (107). Treatment with supraphysiologic doses of GIP during hyperglycemia has been shown to augment insulin secretion in normal (108,109) but not in diabetic humans (100,109).
Although cholecystokinin has the ability to augment insulin secretion in humans, whether it is an incretin at physiologic levels has not been firmly established (110,111,112,113). Its effects are also seen largely at pharmacologic doses (114).
Although cholecystokinin has the ability to augment insulin secretion in humans, whether it is an incretin at physiologic levels has not been firmly established (110,111,112,113). Its effects are also seen largely at pharmacologic doses (114).
The postprandial insulin secretory response may also be influenced by other intestinal peptide hormones, including vasoactive intestinal polypeptide (115), secretin (116,117,118,119), and gastrin (116,120), but the precise role of these hormones remains to be elucidated.
The hormones produced by pancreatic α- and β-cells also modulate insulin release. While glucagon has a stimulatory effect on the β-cell (121), somatostatin suppresses insulin release (122). It is currently unclear whether these hormones reach the β-cell by traveling through the islet-cell interstitium (thus exerting a paracrine effect) or through islet-cell capillaries. Indeed, the importance of these two hormones in regulating basal and postprandial insulin levels under normal physiologic circumstances is in doubt. Other hormones that exert a stimulatory role on insulin secretion include growth hormone (123), glucocorticoids (124), prolactin (125,126,127), placental lactogen (128), and the sex steroids (129). While all of the above hormones may stimulate insulin secretion indirectly by inducing a state of insulin resistance, some also may act directly on the β-cell, possibly to augment its sensitivity to glucose. Thus, hyperinsulinemia is associated with conditions in which these hormones are present in excess, such as acromegaly, Cushing syndrome, and the second half of pregnancy. Furthermore, treatment with placental lactogen (130), hydrocortisone (131), or growth hormone (131,132) is effective in reversing the reduction in insulin response to glucose that is observed in vitro after hypophysectomy. Although hyperinsulinemia following an oral glucose load has been observed in patients with hyperthyroidism (133,134), the increased concentration of immunoreactive insulin in this setting may reflect elevations in serum proinsulin rather than a true increase in serum insulin (135).
Neural Factors
The islets are innervated by both the cholinergic and adrenergic limbs of the autonomic nervous system. While both sympathetic and parasympathetic stimulation enhance secretion of glucagon (136,137), the secretion of insulin is stimulated by vagal nerve fibers and inhibited by sympathetic nerve fibers (136,137,138,139,140,141). Adrenergic inhibition of the β-cell appears to be mediated by the α-adrenoceptor, since its effect is attenuated by the α-antagonist phentolamine (137) and reproduced by the α2-agonist clonidine (142). There is also considerable evidence that many indirect effects of sympathetic nerve stimulation play a role in regulation of β-cell function via stimulation or inhibition of somatostatin, β2 adrenoceptors, and the neuropeptides galanin and neuropeptide Y (143). Parasympathetic stimulation of islets results in stimulation of insulin, glucagon, and pancreatic polypeptide directly and via neuropeptides vasoactive intestinal peptide, gastrin-releasing polypeptide, and pituitary adenylate cyclase-activating polypeptide (143). In addition, sensory innervation of islets may contribute to the regulation of insulin secretion. Sensory nerves have been shown to contain calcitonin gene-related peptide (144,145,146), which may play a inhibitory role, and substance P, whose role is not clearly defined (147,148). The importance of the autonomic nervous system in regulating insulin secretion in vivo is unclear. Studies in animals (149,150) and humans (151,152) have emphasized the importance of the cephalic phase of insulin release—that occurring at the sight, smell, and expectation of food—in regulating the postprandial glucose response. It has been suggested that this reflex, which is under vagal control (96,153), may have a key role in minimizing the early increase in glucose levels following meals (152). Because cholinergic agonists increase the response of the β-cell to glucose in vitro (154), this may be the mechanism by which vagal stimulation achieves its effect. Decreased glucose tolerance following vagotomy has been reported in human subjects (155,156) and following islet denervation in rats (157,158), whereas the insulin secretory response to meals is delayed in patients who have undergone pancreatic transplantation (159). However, many of these patients remain euglycemic without therapy after transplantation (159,160,161,162). Therefore, the importance of the parasympathetic nervous system in maintaining glucose tolerance is unclear. For similar reasons, doubts exist about whether the sympathetic nerve fibers innervating the islets exert a major influence on the basal or postprandial insulin secretory responses. Sympathetic innervation of islets likely accounts for the inhibition of insulin secretion and increased glucagon secretion during exercise and in response to hypoglycemia (163,164,165,166). Similarly, inhibition of insulin secretion mediated by the sympathetic nervous system may account in part for the deteriorating glycemic control reported in individuals with diabetes mellitus who are under severe stress (138,167). The relative contributions of the sympathetic and parasympathetic innervation of the pancreas to the hyperinsulinemia of obesity have been studied, but no consistent differences from lean subjects have been found (168,169,170,171,172).
The neural effects on β-cell function cannot be entirely dissociated from the hormonal effects, since some of the neurotransmitters of the autonomic nervous system are in fact hormones. Furthermore, the secretion of insulinotropic hormones such as GIP and GLP-1 postprandially has been shown to be under vagal (173,174) and adrenergic (175,176) control.
TEMPORAL PATTERN OF INSULIN SECRETION
It has been estimated that, in any 24-hour period, 50% of the total insulin secreted by the pancreas is secreted under basal conditions, and the remainder is secreted in response to meals (177,178). The estimated basal insulin secretion rates typically range from 18 to 32 units per 24 hours (0.7 to 1.3 mg) (34,36,42,177). Moreover, the secretion of insulin is pulsatile, with major pulses being observed every 1.5 to 2 hours (178,179,180,181,182) (Fig. 7.2). These ultradian pulses are present in the basal state but are amplified postprandially (178,179). These pulses have also been observed in subjects receiving glucose intravenously (180,182), suggesting that they are not dependent on food ingestion and are not generated by intermittent absorption of nutrients from the gut. Furthermore, they do not appear to be related to fluctuations in glucagon or cortisol levels (180). Many of these insulin and C-peptide pulses are synchronous with pulses in glucose levels (178,180,182). The ability of an exogenous oscillatory glucose infusion to entrain these pulses in insulin secretion has been shown to indicate normal β-cell function (see Fig. 7.9) (183,184,185). Experimental evidence from studies of animals and humans suggests that superimposed on these large-amplitude ultradian pulses are more rapid oscillations in β-cell activity that occur at a periodicity of 8 to 16 minutes (186,187,188,189,190,191,192). These rapid oscillations in insulin and C-peptide levels do not appear to be coupled as tightly as the ultradian pulses to changes in glucose levels (189,191,193,194). The frequency of these rapid oscillations varies from study to study, and wide variability among subjects is seen even within studies. Accordingly, the physiologic significance of these rapid oscillations in the peripheral circulation is unclear. Although the amplitude of the rapid oscillations is very low in the peripheral circulation, it is much greater in the portal circulation (Fig. 7.3), where these
rapid oscillations may have an important biologic function (194). In this regard, it is possible that the liver responds more favorably to insulin delivered in a pulsatile fashion than to insulin delivered at a constant rate (195,196,197).
rapid oscillations may have an important biologic function (194). In this regard, it is possible that the liver responds more favorably to insulin delivered in a pulsatile fashion than to insulin delivered at a constant rate (195,196,197).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree