Angiogenic Switch
The normal microenvironment has an anti-angiogenic disposition. As tumors grow they are initially dependent on the normal microenvironment for oxygen and other nutrients. However, since the diffusion limit of oxygen is around 100 μm, as tumors expand, those cells that are no longer within the oxygen diffusion perimeter become hypoxic.
6 In the setting of hypoxia, multiple intracellular molecules are activated. One well-characterized result of hypoxia is the activation of the hypoxia inducible factor-1 (HIF-1) transcriptional complex.
7 Activation of this complex leads to up-regulation of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and nitric oxide synthase (NOS), which are released into the microenvironment. Consistent with this finding, HIF-1 is up-regulated in many cancers.
7 However, like most pathways in oncogenesis, redundancy is present and the HIF system is also influenced by many oncogenic pathways, including the insulin-like growth factor-1, epidermal growth factor (EGF), mutant Ras, and Src kinase pathways as well as tumor suppressor mutations, including PTEN, p53, p14ARF, and pVHL (von Hippel-Lindau).
7 The activation of HIF-1 is a critical step in initiation of the “angiogenic switch” in which the normal microenvironment changes from anti-angiogenic to pro-angiogenic and new blood vessel formation is started. Drugs targeting HIF-1 have been developed for clinical use and are currently being tested in early phase trials.
The Vascular Endothelial Growth Factor (VEGF) and VEGF Receptor Family
Activation of the VEGF pathway is critical in both physiologic and pathologic angiogenesis. The complexities of this system are beyond the scope of this chapter; therefore, only a brief overview is provided. Detailed reviews are available elsewhere.
8,
9,
10 The VEGF receptor family is made up of three tyrosine kinase receptors. Of these, only VEGFR-1 and VEGFR-2 bind the main ligand important in tumor angiogenesis, VEGF-A.
8,
10 VEGF-A, usually referred to as VEGF, belongs to a family of genes including placenta growth factor (PLGF), VEGF-B, VEGF-C, and VEGF-D. The biology of VEGF and its primary receptors, VEGFR-1 and VEGFR-2, will be discussed in this chapter (
Table 26-1).
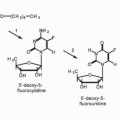
The two circulating isoforms of VEGF, VEGF
121 and VEGF
165, are the major mediators of tumor angiogenesis.
9 One of the main inducers of VEGF expression is tumor hypoxia.
11 This is mediated in part by HIF-1.
12 Interestingly, loss of the von Hippel-Lindau (
vHL) tumor suppressor gene in renal cell carcinomas results in constitutive overexpression of HIF-1 leading to elevated VEGF expression in these tumors.
13 Several other major growth factors including EGF, transforming growth factors alpha (TGF-
α) and beta (TGF-
β), keratinocyte growth factor, insulin-like growth factor-1, fibroblast growth factor (FGF), and PDGF also have been shown to up-regulate VEGF mRNA expression.
8 In addition, oncogenic mutations or amplifications of the Ras oncogene and inflammatory cytokines (IL-1
α and IL-6) are associated with VEGF gene induction.
14,
15 VEGF production can also originate from tumor-associated stromal cells
9 suggesting that paracrine or autocrine release of these factors cooperate with hypoxia to induce tumor angiogenesis via VEGF upregulation. Vascular permeability is also mediated by VEGF, which forms fenestrations in blood vessels.
10 Therefore, agents targeting VEGF are also thought to exert antitumorigenic effects by allowing improved delivery of other agents, such as chemotherapy, to tumors.
16The VEGF-1 and VEGF-2 receptors are expressed normally on vascular endothelial cells engaged in angiogenesis as well as on bone marrow-derived cells. In addition, many solid and hematologic tumors also express the VEGF receptors, primarily VEGFR-1.
9 These receptors have a similar protein structure with seven immunoglobulin-like domains in the extracellular region, a single transmembrane region, and a consensus tyrosine kinase sequence that is interrupted by a kinase-insert domain.
8 Interestingly, the VEGF receptors can also be expressed inside the cell, where they can promote cell survival by an “intracrine” mechanism.
9VEGFR-1 (also known as Flt-1) was the first identified VEGF receptor; however, its role in angiogenesis remains controversial (
Table 26-1). The data suggest that the function of VEGFR-1 differs depending on the developmental stage of the animal, the cell type on which it is being expressed, and the ligand to which it is binding (VEGF-A, VEGF-B, or PLGF).
8,
9 Studies linking VEGFR-1 to angiogenesis have found that expression of VEGFR-1 is up-regulated by hypoxia. Similar to the VEGF, this is mediated by HIF-1. However, VEGFR-1 has also been identified as a negative regulator of VEGF, especially in embryonic development. In addition, its signal transduction properties are very weak. In general, VEGFR-1 is thought to have little role in tumor angiogenesis.
VEGFR-2 (also known as KDR or Flk-1) is the main regulator of endothelial cell proliferation and survival, as well as vascular permeability (
Table 26-1).
10 Upon VEGF ligand binding, the receptor undergoes dimerization and tyrosine phosphorylation. In endothelial cells, this VEGFR-2 activation leads to initiation of several signaling cascades.
9 This includes activation of the mitogenactivating protein kinase (MAPK) pathway, which involves phospholipase C-
γ (PLC
γ), protein kinase C (PKC), Raf kinase, and MEK. Activation of this pathway results in DNA synthesis and cell growth. Other pathways initiated by VEGFR-2 activation are the phosphatidylinositol 3′-kinase (PI3K)/Akt pathway that leads to increased endothelial cell survival and the Src family pathway that results in cell migration. Many of these pathways are abnormally expressed in tumors and have been targeted with specific inhibitors. In addition, the neuropilin (NRP) receptors also bind to VEGF and can act as co-receptors with VEGFR-2 to regulate angiogenesis; therefore, these receptors may be targeted by novel agents in the future.
9
Other Molecular Pathways Important in Tumor Angiogenesis
Matrix metalloproteinases (MMPs) are a family of structurally related zinc-containing endopeptidases that are involved in the degradation of extracellular matrix components (ECM).
17 In angiogenesis, MMPs mediate remodeling and invasion of the ECM by new vessels by regulating endothelial cell attachment, proliferation, and migration. These observations suggest that matrix metalloproteinase inhibitors (MMPIs) could inhibit tumor progression at both the primary tumor site and sites of metastases. Several MMPIs have been developed for clinical use, but results in phase III clinical trials of patients with advanced malignancy were disappointing.
18,
19Interaction between endothelial cells and the extracellular matrix is critical to tumor angiogenesis. The integrins are transmembrane receptors that bind to extracellular matrix proteins and play a significant role in this interaction. Studies have implicated a number of endothelial cell integrins in the regulation of endothelial cell growth, survival, and migration during angiogenesis.
20 In addition, integrin signaling is dysfunctional in cancer cells and their expression may correlate with prognosis.
21 Several inhibitors of these endothelial cell integrins have been developed and tested in early phase clinical trials.
20 Clinical studies indicate signs of potential therapeutic benefit from these agents; however, further testing is still needed.
Another receptor tyrosine kinase pathway involved in tumor angiogenesis involves the tie-2 receptor.
9 This receptor is expressed principally on the vascular endothelium. Its major ligands are angiopoietin-1 (ang-1) and angiopoietin-2 (ang-2). In concert with VEGF, this pathway stabilizes and matures new capillaries. Ang-1, ang-2, and tie-2 expression have been correlated with prognosis in several cancers, including early stage bladder cancers and breast
cancer.
22,
23 Peptibodies against ang-2 have been developed and are in early clinical development.
Another pathway that also appears to play an important role in tumor angiogenesis is the notch receptor pathway. The notch receptors are cell surface receptors implicated in cell fate, differentiation, and proliferation.
9,
24 The ligands for these receptors are the transmembrane proteins, jagged and delta-like ligand (Dll), which are expressed on adjacent cells. Vascular endothelial cells express notch 1 and 4, as well as jagged 1, Dll-1, and Dll4. Notch-Dll4 signaling is essential for vascular development in the embryo and Dll4 is up-regulated in tumor vasculature. This is thought to be in part VEGF-mediated. Novel agents targeting this pathway are also currently in early phase clinical development.