Platelet aggregation is essential for the formation of a hemostatic platelet plug.
129 In contrast to platelet adhesion, platelet aggregation is an active metabolic process, requiring platelet stimulation by agonists such as thrombin, collagen, and ADP and exposure of a binding site for fibrinogen or vWF on the integrin
αIIbβ3.
130,131,132 αIIbβ3-bound fibrinogen or vWF then crosslinks adjacent platelets into an occlusive plug.
αIIbβ3, like other members of the integrin family, resides on the cell surface in an equilibrium between inactive (low affinity) and active (high affinity) conformations.
133,134 Platelet stimulation shifts
αIIbβ3 to its active conformation, enabling it to bind ligands and mediate platelet aggregation.
134 Platelet stimulation also enables interaction of the
αIIbβ3 cytoplasmic tails with submembranous actin filaments, providing a link between the force of cytoskeletal contraction and a fibrin clot, resulting in clot retraction.
135,136αIIb itself has an unreduced molecular weight of 136,000 and dissociates into a 125,000 mol. wt. heavy chain (
αIIbα) and 23,000 mol. wt. light chain (
αIIbβ) following disulfide bond reduction.
137 αIIb is synthesized as single chain precursor (Pro-GPIIb) in the rough endoplasmic reticulum (ER)
140 where it associates with
β3. The resulting heterodimer is transported to the Golgi complex where Pro-
αIIb is cleaved into heavy and light chains.
141 In contrast to
αIIb,
β3 is a single chain protein containing 56 cysteine residues and 28 disulfide bonds
142; its apparent molecular weight of 90,000 on unreduced sodium dodecyl sulfate gels increases to 110,000 following disulfide bond reduction.
137 The disulfide bonds in
β3 are concentrated in three regions of the extracellular portion of the molecule: a cysteine-rich, proteaseresistant amino terminus (residues 1 to 62), a protease-sensitive central region (residues 101 to 422), and a disulfide-rich, protease-resistant core (residues 423 to 622).
143
Laboratory Findings
Platelet counts and platelet morphology on peripheral blood smears are normal. The bleeding time of affected individuals is markedly prolonged.
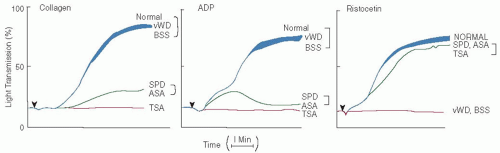
153 A diagnosis of GT is usually suspected when platelet aggregometry reveals absent agonist-stimulated platelet aggregation (see
FIGURE 65.1). Platelet secretion induced by strong agonists such as thrombin is normal, but secretion in response to weak agonists such ADP and epinephrine which requires platelet aggregation does not occur. Coagulation tests, such as the prothrombin time and the partial thromboplastin time, are normal in GT, whereas clot retraction in the presence of GT platelets is absent or reduced.
In addition to their inability to aggregate, GT platelets do not spread normally on the subendothelial matrix because of an impaired ability to interact with fibronectin and vWF in the matrix.
154 The amount of fibrinogen in the
α-granules of GT platelets is decreased to absent.
127 Human megakaryocytes do not synthesize fibrinogen, but rather, it is derived from plasma by an
αIIbβ3-mediated endocytic process.
155,156,157,158,159 A number of biochemical reactions in platelets that require the presence of
αIIbβ3 are impaired in GT platelets. For example, the tyrosine phosphorylation of multiple intracellular signaling proteins depends on ligand binding to
αIIbβ3 and does not occur in GT platelets.
160 Calpain, a calcium-dependent thiol protease, is activated when stirred normal platelets are stimulated by thrombin, but not when GT platelets are treated in a similar manner.
161 The kinetics of calcium exchange in unstimulated GT platelets is inexplicably decreased
162 since it is not clear what role, if any,
αIIbβ3 plays in calcium transport.
163,164 Clot retraction in blood containing GT platelets is either absent or reduced.
127,135Aspects of platelet function that do not depend on
αIIbβ3 are normal in GT. GT platelets interact normally with collagen
153 and undergo secretion when stimulated by “strong agonists” such as thrombin.
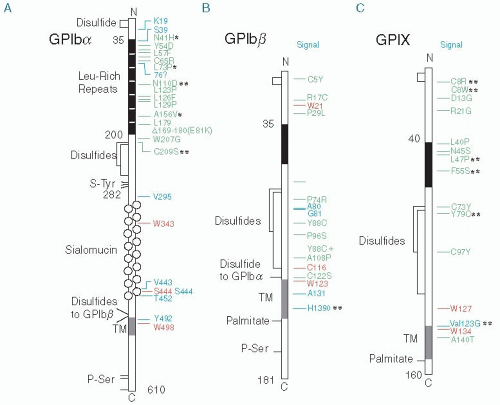
165 Incubation of GT platelets with ristocetin induces vWF binding to GPIb-IX-V
10 and GT platelets adhere to vWF in the subendothelium.
154 GT platelets also express normal amounts of procoagulant activity following lysis.
50 However, agonist-stimulated prothrombinase activity varies and is influenced by the nature of platelet agonist.
50,166
Genetics
The genes for
αIIb and
β3 are located on the long arm of chromosome 17 at q21→23
167 with the
αIIb gene at minimum distance of 365 kb downstream from the
β3 gene.
168 The
αIIb gene (
ITGA2B) spans ≈18 kb and consists of 30 exons ranging in size from 46 to 220 bp.
169 Like the genes for other proteins expressed in megakaryocytes, its 5′ flanking region lacks TATA or CAAT boxes. Instead, it contains a linear array of regulatory elements that includes two motifs recognized by the GATA-1 transcription factor and its cofactor FOG
170,171; sequences flanking each GATA-1 binding site are recognized by Fli-1 and perhaps other members of the Ets family of transcription factors
172,173,174,175; and an Sp1-binding silencer element is located between the GATA-1 sites.
176 The gene for
β3 (
ITGB3) spans 63 kb and contains 15 exons.
151,177 Like the
αIIb gene, the 5′ flanking region of the
β3 gene lacks TATA or CAAT boxes.
178GT is an autosomal recessive disorder with disease clusters in populations where consanguinity is common. It has been subclassified into three types based on the amount of
αIIbβ3 present per platelet and the presence or absence of
α-granule fibrinogen and clot retraction.
127 In type I, platelets contain <5% of the normal amount of
αIIbβ3 and clot retraction and
α-granule fibrinogen are absent. In type II, platelets contain 10% to 20% of the normal amount of
αIIbβ3, clot retraction is decreased, and
α-granule fibrinogen is present. In “variant” thrombasthenia, the platelet content of
αIIbβ3 is ≥50% of normal, indicating that the
αIIbβ3 abnormality is qualitative, rather than quantitative.
Because the expression of
αIIbβ3 on the platelet surface requires the formation of correctly folded heterodimers,
179 mutations in the genes for either
αIIb or
β3 can produce GT. Approximately 200 different mutations responsible for GT have been identified
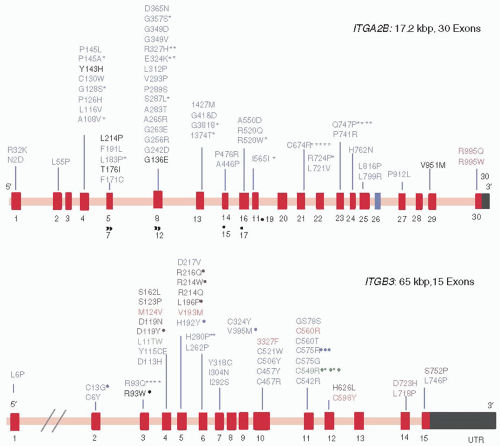
180 (
FIGURE 65.3). The vast majority of these mutations consist of a mix of missense and nonsense mutations, nucleotide deletions and insertions, and alternative splicing, resulting in type I and type II GT. However, the most informative mutations have been qualitative
αIIbβ3 abnormalities (variant GT) that primarily perturb
αIIbβ3 function rather than the level of
αIIbβ3 expression.
Mutations located in the extracellular portion of
αIIbβ3 cause GT by either impairing fibrinogen or vWF binding to the active
αIIbβ3 conformation or by paradoxically inducing constitutive
αIIbβ3 activation but causing GT by concurrently decreasing the amount of
αIIbβ3 expressed on the platelet surface. Six missense mutations (the
αIIb mutation Tyr143His
181 and the
β3 mutations Asp119Tyr,
182 Val193Met,
183 Arg214Trp,
184,185 Arg214Gln,
186 and Aspr217Val
187) are located in regions implicated in ligand binding to
αIIbβ3 and cause GT by preventing ligand binding.
188,189 Similarly, an insertion of two amino acids (Arg-Thr) into the Cys146-Cys167 loop of
αIIb produced an inactive
αIIbβ3 heterodimer.
190 An eighth mutation,
β3 Ser123Pro, did not impair
αIIbβ3 expression or agonist-induced ligand binding but was associated with absent ADP- and collagen-induced platelet aggregation.
191 The
β3 mutation, Leu196Pro, was identified independently in two French patients and severely restricted
αIIbβ3 expression.
192,193 However, the residual
αIIbβ3 that made it to the platelet surface was unable to bind ligands, presumably because the mutation prevents
αIIbβ3 from assuming its active conformation. By contract, the
β3 mutation Cys560Arg locked
αIIbβ3 in a high-affinity conformation, but like Leu196Pro, it likely produced a GT-phenotype because the small amount of constitutively active
αIIbβ3 on the platelet surface was insufficient to support platelet aggregation.
194The extracellular stalks of
αIIbβ3 consist of the thigh, calf-1, and calf-2 domains of
αIIb and the epidermal growth factor (EGF)-like domains 1 to 4 and the
β TD domain of
β3.
195 The distal portions of the stalks form an interface containing interacting energetic “hot spots” that are critical for maintaining
αIIbβ3 in a stable, inactive conformation.
196,197 Thus, it is not surprising that four mutations involving cysteine residues located at the third and fourth EGF domains of
β3 (Cys49Arg,
198 Cys560Phe,
199 Cys560Arg,
194 and Cys598Tyr
199,199a) as well as a Gly579Ser mutation, not only resulted in type II thrombasthenia but also induced constitutive ligand-binding activity in the residual
αIIbβ3. Similarly, a Ser527Phe mutation detected in the third
β3 EGF domain of a patient who presented with a mild bleeding diathesis was found to cause constitutive activation of recombinant
αIIbβ3 expressed in Chinese hamster ovary cells.
200Naturally occurring mutations involving the
β3 cytoplasmic domain have confirmed the singular importance of this structure in regulating
αIIbβ3 function. The first mutation identified, a missense mutation that results in the substitution of Pro for Ser at residue 752, prevents
αIIbβ3 activation by cellular agonists,
201,202,203 likely because it abrogates binding of the cytoplasmic protein kindlin-3 to
β3 (see below).
204 A second missense mutation, identified separately in two unrelated patients,
205,206 converts the Arg724 codon into a stop codon, resulting in the synthesis of a truncated
β3 molecule lacking 39
C-terminal amino acids. As a consequence,
αIIbβ3 is unable to transduce both “inside-out” and “outside-in signals,” likely because
β3 is now unable to interact with both kindlin-3 and talin.
204 A third mutation, identified in an Palestinian Arab with GT, produces aberrant mRNA splicing at the intron 14/exon 15 junction and replacement of the
β3 sequence distal to residue 741 with 40 unrelated amino acids.
207 This results in the absence of the distal talin binding site in the
β3 cytoplasmic domain, as well the binding site for kindlin-3, and an
αIIbβ3 extracellular domain that appears to be “locked” in its inactive conformation.
208Lastly, several heterozygous mutations located in the highly conserved membrane-proximal regions of the
αIIb and
β3 cytoplasmic domains have been associated with GT-like phenotypes and macrothrombocytopenia.
209 Thus, replacement of
αIIb Arg995 with either Gln
209,210 or Trp
211 and
β3 Asp723 with His
212,213 results in decreased, but not absent, platelet aggregation, an approximate 50% decrease in the total amount of platelet
αIIbβ3 predominantly affecting the surface—rather than the granule—membrane pool, a moderate degree of macrothrombocytopenia and platelet “anisocytosis,” and partial
αIIbβ3 activation when
αIIbβ3 is expressed in tissue culture cells. A
β3 Leu718Pro mutation produced a similar phenotype,
214 as did a two residue deletion in the
β TD domain of
β3.
215 How these mutations in
αIIbβ3 produce this spectrum of abnormalities is not clear since “classic” GT is not associated with alterations in platelet number or size, but the association of macrothrombocytopenia with BSS and the MYH9 disorders suggests that perturbed
αIIbβ3-cytoskeleton interactions could be involved.
Differential Diagnosis
A history of lifelong bleeding, a prolonged bleeding time, and absent platelet aggregation are diagnostic of GT and differentiate it from other disorders of platelet adhesion and secretion. Rare instances of acquired GT have been reported and can be differentiated from congenital GT by history. Although autoantibodies against
αIIbβ3 are frequently detected in patients with idiopathic thrombocytopenic purpura,
216,217 they rarely induce a GT-like state.
218,219,220,221 There are anecdotal reports of autoantibodies producing acquired GT in patients with Hodgkin and non-Hodgkin lymphoma, hairy cell leukemia, and after an allergic reaction to diclophenac.
222,223,224 A patient with multiple myeloma has also been reported whose IgG
1κ paraprotein was directed against
β3 and inhibited
αIIbβ3 function.
225 Congenital afibrinogenemia may be associated with a prolonged bleeding time and decreased
in vitro platelet aggregation due to the absence of sufficient fibrinogen to support normal platelet aggregation.
226In the LAD-III/LAD-1/variant syndrome, patients manifest mucocutaneous bleeding and absent platelet aggregation, despite normal or nearly normal
αIIbβ3 expression.
227,228,229 Concurrently, they suffer from recurrent and severe bacterial infections due to defective leukocyte integrin function, implying that the syndrome results from a general defect in integrin activation. Although initially attributed to defective expression of the Rap-1 activator CalDAG-GEFI,
229,230,231 in several kindreds,
232,233 mutations have been identified in the
KINDLIN3 gene encoding the protein kindlin-3 that binds to
β1,
β2, and
β3 integrin cytoplasmic tails and is required for agonist-stimulated integrin activation.
204,234,235
Therapy
The mainstay of treatment of bleeding in GT remains the transfusion of normal platelets.
111 Because bleeding is a lifelong problem, use of HLA-matched platelets should be considered to lessen the chance of refractoriness to transfusion due to platelet alloimmunization. Patients with GT, especially patients with null mutations, not infrequently develop alloantibodies against normal
αIIbβ3 after transfusion, potentially limiting the effectiveness of transfused platelets.
236,237,238,239 Protein A-Sepharose immunoadsorption has been reported to restore the efficacy of transfused platelets in this situation.
240 Oral contraceptives have been useful in controlling menorrhagia. Regular dental care is essential in minimizing gingival bleeding, and fibrinolytic inhibitors, in addition to platelet transfusions, may be useful in controlling bleeding after dental extractions. Corticosteroids are not efficacious in managing bleeding in thrombasthenic patients,
153 and there is little evidence that desmopressin (DDAVP) is useful.
114,241 Recombinant factor VIIa has been found to be efficacious for the treatment of bleeding episodes and for obtaining surgical hemostasis in patients with GT, particularly in patients who have developed alloantibodies from prior platelet transfusions.
111,242,243 The GT phenotype of several severely affected individuals has been corrected by bone marrow,
244,245,246,247 stem cell,
248,249 and unrelated donor cord blood bone marrow transplantation.
250 Lastly, platelet gene therapy has improved hemostatic function in
αIIbβ3-deficient dogs,
251 suggesting that at some point in the future, gene therapy could be used in humans.