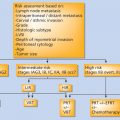
Inflammation and cancer
Jelena Todoric, MD, PhD  Atsushi Umemura, MD, PhD
Atsushi Umemura, MD, PhD  Koji Taniguchi, MD, PhD
Koji Taniguchi, MD, PhD  Michael Karin, PhD
Michael Karin, PhD
Overview
Epidemiological studies and experimental evidence have provided strong support to the long standing notion that chronic inflammation stimulates the development and progression of malignant neoplasms. It is now clear that pre-existing inflammation caused by persistent viral and microbial infections, autoimmune diseases, environmental irritants, and even obesity promote tumor development and may account for up to 20% of all cancer deaths. Inflammation also appears subsequent to cancer development and such “tumor-elicited inflammation” plays a key role in the malignant progression and metastatic dissemination of most cancers. Although some common anti-inflammatory drugs, such as aspirin, substantially reduce cancer risk, there is still an unmet need for the clinical development of new therapeutics that target cancer-associated inflammation.
Chronic inflammation and cancer
A potential link between inflammation and cancer was first proposed by the German pathologist, Rudolf Virchow, in the nineteenth century. Virchow supposedly detected immune cell infiltrates in solid tumors, leading him to suggest that inflammation could be a cause of tumorigenesis. Although largely forgotten for more than a century, the interest in the link between inflammation and cancer was rekindled by epidemiological studies suggesting that underlying chronic inflammation is associated with nearly 20% of cancer deaths. More recently, solid evidence has accumulated pointing to inflammation as a critical hallmark of cancer, and some of the key underlying molecular mechanisms were elucidated.1 Furthermore, it has become clear that an inflammatory microenvironment is an important component of most cancers, including some hematopoietic malignancies, even in tumors where a direct causal relationship is yet to be established.2 Notably, only 10% of all cancers are linked to germ line mutations, whereas the vast majority (90%) are caused by acquired somatic mutations, many of which may be caused by environmental factors that are often associated with chronic inflammation. However, in most cases, chronic inflammation caused by persistent infections (Table 1) and autoimmune disease acts as a tumor promoter.3 One of the most notable examples is inflammatory bowel disease (IBD), such as ulcerative colitis, which greatly increases the risk of colorectal cancer (CRC).3 Similarly, persistent Helicobacter pylori infection causes chronic gastritis and can lead to gastric cancer. Infections with hepatitis B virus (HBV) or hepatitis C virus (HCV) give rise to hepatitis (chronic liver inflammation), eventually culminating in liver cancer (hepatocellular carcinoma or HCC). About 85% of the current HCC cases, one of the leading causes of cancer death worldwide, are derived from chronic liver damage caused by either HBV or HCV infections.
Table 1 Major cancer sites and types associated with infectious agents
| Infectious agent | Cancer site and type |
| Helicobacter pylori | Gastric cancer |
| Hepatitis B viurs (HBV), hepatitis C virus (HCV) | Liver cancer |
| Human papillomavirus (HPV) | Cervical cancer; anogenital cancer; oropharynx cancer |
| Epstein–Barr virus (EBV) | Nasopharynx cancer |
| Schistosoma haematobium | Bladder cancer |
| Human immunodeficiency virus (HIV) | Non-Hodgkin lymphoma; Kaposi sarcoma; cervical cancer |
As of 2008, around 16% of cancer cases worldwide are attributed to infectious agents. The most important players are Helicobacter pylori, HBV and HCV, and HPV.
Source: de Martel 2012.20 Reproduced with permission of Elsevier.
In the past generation, obesity has become a major public health problem in most developed countries, increasing the risk of various diseases, including cancer. Obesity induces low-grade, sustained inflammation that influences many organ systems and is currently one of the most common risk factors for almost all types of cancer. Inversely, long-term use of anti-inflammatory drugs, such as aspirin or selective cyclooxygenase-2 (COX-2) inhibitors, results in a substantial reduction in the risk of cancer development. These results further underscore the importance of inflammation as one of the major causes of cancer and a driver of malignant progression and metastatic dissemination.
It is important to note that only in some types of cancer is chronic inflammation present before malignant transformation or tumor initiation, but in the majority of cancers, oncogenic and neoplastic changes induce a localized inflammatory microenvironment that further enhances tumor development and progression. Understanding the links between inflammation and cancer is essential for the development of better preventative and therapeutic strategies.
Inflammatory cells, the microenvironment, and cancer
Macrophages
Macrophages are the most abundant immune cells in the tumor microenviroment. Tumor-associated macrophages (TAMs) aquire protumorigenic properties in both primary and metastatic sites4 and play several supportive roles in cancer development and progression. The tumor-promoting functions of TAMs affect cancer cell proliferation and survival, angiogenesis, cancer cell invasion, motility, intravasation and extravasation, as well as suppression of cytotoxic T-cell responses.5, 6 During tumor initiation/early promotion, TAMs secrete cytokines and growth factors that stimulate the proliferation and survival of initiated epithelial cells bearing oncogenic mutations.7 It is not clear whether, at this early stage of tumor development, macrophages can eliminate abberant cells before they undergo polarization to acquire tumor-promoting properties. Two major subsets of macrophages were described: classically activated (M1) and alternatively activated (M2).8 M1 macrophages express and secrete a variety of proinflammatory cytokines, chemokines, and effector molecules, including IL-12, IL-23, tumor necerosis factor (TNF), and iNOS, whereas M2 macrophages release anti-inflammatory mediators, such as IL-10, TGF-β, and arginase-1. During early tumor progression, several factors cause a phenotypic switch in TAM polarization to the MS phenotype, thereby providing an immunosuppressive microenvironment that is permissive for tumor growth. Exposure of macrophages to IL-4 produced by CD4+ T cells and/or cancer cells,5, 9 growth factors such as colony-stimulating factor-1 (CSF1),10 GM-CSF,11 and TGF-β secreted by cancer cells induces the tumor-promoting M2 phenotype, but the actual origin of TAMs is still a matter of debate. Most TAMs may be derived from Ly6C+ circulating monocytes,12–14 but the classical view of the bone marrow (BM) as the major site of monocyte production has been challenged by recent studies suggesting that extramedullary hematopoiesis sustains a reservoir of tumor-infiltrating monocytes.15 However, lineage tracing experiments demonstrated that the splenic contribution is minor and that the BM is still the major source of monocytes that give rise to TAMs, at least in some tumor models.16 In addition to its ability to support TAM proliferation and activation, CSF1 is the major lineage regulator and a chemotactic factor for macrophages.17 Blood vessels that provide oxygenation and nutrition dramatically increase in most tumors during malignant conversion, a process often referred to as the “angiogenic switch.” TAMs that express TIE2 regulate this process mostly via production of vascular endothelial growth factor (VEGF).18 TIE2+ macrophages also promote cancer cell migration and intravasation into the circulation.19 Furthermore, immunsuppression in the tumor bed is partially mediated by macrophages that contribute to the inhibition of cytotoxic CD8+ cells, which are discussed further in the following sections (Table 1).
T and B lymphocytes
Many tumors express antigens that can be recognized by T lymphocytes, and analysis of the tumor microenviroment in a variety of solid tumors has revealed the presence of T-cell infiltrates. However, despite an active immune response in a subset of patients, including infiltration with cytotoxic CD8+ T cells, tumors that progress are obviously not rejected. This indicates the existence of immunosuppressive mechanisms that counteract anticancer immunity. One such mechanism depends on accumulation of CD4+Foxp3+ regulatory T cells (Treg) that play a pivotal role in maintenance of immunological self-tolerance.21 Furthermore, Treg can also produce cytokines, such as RANK ligand (RANKL) that promote tumor progression, as first demonstrated in breast cancer.22 The CD8+ T-cell response in tumors involves the participation of stress-associated or damage-associated molecular patterns (DAMP), through which tissue injury or cancer cell death can promote immunity. Production of type I interferon by CD8α+ dendritic cells (DCs) also favors the activation of anticancer immunity.23 DCs and macrophages express Major histocompatibility complex (MHC) class I molecules that present antigens to cytotoxic CD8+ T cells. However, macrophages that express membrane-bound or soluble forms of histocompatibility leukocyte antigen (HLA) molecules can directly inhibit activation of natural killer (NK) cells and other T-cell subsets.24 In addition, HLA-G and HLA-E can inhibit NK-cell secretion of Interferon IFN-γ, an important mediator of CD8+ T-cell activation.25 Activation of the inhibitory receptors programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) by ligands that are expressed on either cancer cells or immune cells controls the intensity of immune response and inhibits T-cell receptor (TCR) and B-cell receptor (BCR) signaling.26 TAMs have been shown to upregulate PD-1 ligand expression in response to hypoxia inducible factor 1α (HIF-1α) in hypoxic tumor regions, leading to T-cell suppression.27 Furthermore, TAMs secrete a variety of cytokines and chemokines that can directly suppress T-cell activation and recruit immunosuppressive Treg cells. Th17 cells are T cells that produce the inflammatory cytokines IL-17A and IL-17F. These cells are recruited into early colon cancers and play an important role in accelerating tumor progression.28 B lymphocytes are also present in the tumor microenvironment. In mouse models of skin cancer or squamous cell carcinoma, B cells promote tumor progression by activating cells and other myeloid cells.29, 30 In prostate cancer, newly recruited B cells promote the development of aggressive castration-resistant tumors by producing the proinflammatory cytokine lymphotoxin.31 Tumor-infiltrating B cells can also respond to the high local concentration of TGF-β present within certain tumors and assume an immunosuppressive phenotype that prevents the activation of cytotoxic T cells.
Cancer-associated fibroblasts (CAFs)
CAFs are fibroblastic cells that reside within the tumor microenviroment. CAFs promote tumorigenesis by stimulating cancer cell proliferation, enhancing angiogenesis, and modifying the architecture of the extracellular matrix (ECM).32–37 In normal tissues, fibroblasts prevent initiation of neoplastic growth through negative regulation of epithelial proliferation by TGF-β-mediated signaling. In contrast, CAFs show proinflammatory and tumor-promoting properties38 and produce a wide variety of chemokines and cytokines including osteopontin (OPN), CXCL1, CXCL2, IL-6, IL1-β, CCL-5, stromal-derived factor (SDF-1α), and TNF.39, 40 In early stages of the tumorigenic process, fibroblasts “sense” changes in tissue architecture that result from increased proliferation of neighboring epithelial cells. This results in activation of proinflammatory signaling in fibroblasts.41–43 Furthermore, the proinflammatory properties of CAFs are enhanced by mediators secreted by resident immune cells.40 For example, B cells produce antibodies that are deposited in the tumor bed owing to small amounts of blood seeping out of leaky blood vessels and can induce secretion of IL-1 by resident immune cells. This in turn drives fibroblasts into a proinflammatory phenotype.44, 45 CAFs can also be affected by tumor hypoxia, which upregulates their ability to produce TGF-β and certain chemokines.46 The major mechanism of tumor promotion by CAFs involves secretion of cytokines and chemokines that recruit immune cells into the tumor microenviroment and alter their function. For example, CAF-secreted CCL2 recruits macrophages to the tumor, whereas CAF-derived immunosuppressive cytokine TGF-β inhibits the function of NK and CD8+ T cells47 while inducing differentiation of Treg cells.48 CAF-derived CXCL13 mediates recruitment of B cells into androgen-deprived prostate cancer, leading to development of castration resistance.46
Pro- and anti-inflammatory cytokines in cancer
Cytokines are small proteins produced and released by many types of cells, especially immune cells, and function as mediators of cell–cell communication via specific membrane receptors.7 Cytokines are usually induced in response to inflammation and provide an important link between inflammation and cancer through cancer-intrinsic (cancer cell proliferation, survival, and invasive properties) and cancer-extrinsic (tumor microenvironment) effects (Figure 1).49 TNF and IL-6 are the best-studied protumorigenic cytokines linking inflammation to cancer and are overexpressed in most cancers (Table 2).50 Several transcription factors, NF-κB, signal transducer and activator of transcription 3 (STAT3), and AP-1, are the major downstream effectors of cytokine signaling and are activated in the majority of cancers, where they cooperate to regulate many cancer-related pathophysiological processes, including cell proliferation and survival, differentiation, immunity, metabolism, and metastatic behavior.51
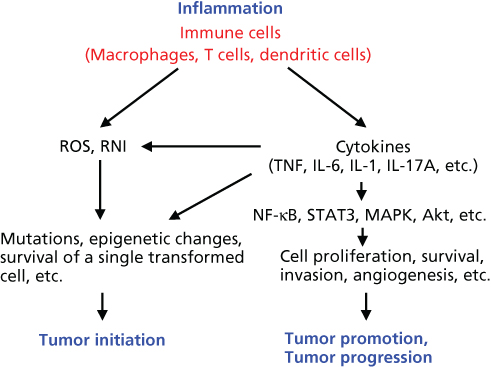
Figure 1 The role of inflammation in tumor initiation, promotion, and progression.
Table 2 Cytokines in inflammation and cancer
| Cytokine | Functions in cancer and immune cells | Pathways |
| TNF | Proinflammatory, cell survival or death | NF-κB, MAPK |
| IL-6 | Proinflammatory, cell proliferation and survival | JAK/STAT3, ERK, Akt |
| IL-11 | Tissue-protective, cell proliferation and survival | JAK/STAT3, ERK, Akt |
| IL-1 | Proinflammatory, activates immune cells | NF-κB, MAPK |
| IL-17A | Proinflammatory, increases inflammation | NF-κB, MAPK |
| IL-12 | Proinflammatory, Th1 differentiation | JAK/STAT4 |
| IL-23 | Proinflammatory, Th17 differentiation | JAK/STAT3, STAT4 |
| IL-10 | Anti-inflammatory, inhibits NF-κB | JAK/STAT3 |
| IL-22 | Tissue-protective, cell proliferation and survival | JAK/STAT3 |
| TGF-β | Anti-inflammatory, dual role in tumorigenesis | Smad, MAPK |
Tumor necerosis factor
TNF is the founding member of a large cytokine family and major activator of inflammatory and immune responses to a variety of pathogens.52, 53 TNF is an important mediator of cachexia and several acute and chronic inflammatory diseases, such as sepsis, rheumatoid arthritis (RA), and IBD. TNF is mainly produced by macrophages, which are activated by pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), via toll-like receptors (TLRs). TNF activates several signaling pathways by binding to TNF receptor (TNFR)1 and/or TNFR2. Activation of these pathways induces production of other inflammatory cytokines, such as IL-6 and IL-1 and, depending on cellular context, modulates cell survival and death. Elevated TNF expression promotes tumorigenesis and metastasis at multiple steps, including cellular transformation, survival, proliferation, invasion, and angiogenesis.52, 53 TNF-induced NF-κB activation enhances β-catenin activation, which leads to dedifferentiation of non-stem cells that acquire tumor-initiating capacity in a mouse model of colorectal cancer.54 Several TNF inhibitors are clinically available and are approved for the treatment of RA and IBD. Although high doses of TNF have been used in the treatment of sarcomas of the extremities, localized production of TNF is associated with tumor promotion.
The IL-6 family of cytokines
IL-6 is another proinflammatory cytokine that activates the acute phase response characterized by expression of C-reactive protein (CRP) and serum amyloid A (SAA).55 IL-6 is also a mediator of cancer cachexia and is one of the best-characterized protumorigenic cytokines.56, 57 The IL-6 family also includes IL-11, IL-27, IL-31, leukemia inhibitory factor (LIF), oncostatin M (OSM), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1), and cardiotrophin-like cytokine (CLC), all of which control cell proliferation, survival, migration, invasion, metastasis, angiogenesis, and inflammation. IL-6 family members activate the Janus kinase (JAK)-STAT3 pathway, the Src homology 2 (SH2)-containing protein tyrosine phosphatase-2 (SHP-2)-Ras-Raf-MEK-extracellular signal-regulated kinase (ERK) pathway, and the phosphoinositide 3-kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway through engagement of their unique receptor(s) that associate with the common signaling subunit gp130. Among these effectors, STAT3 is considered an oncogene and a major downstream mediator of gp130 signaling in cancer.58, 59 IL-6 and IL-11 are produced by many different types of cells, including immune cells, fibroblasts, and epithelial cells, and IL-6, IL-11, and STAT3 are highly expressed in many solid tumors. A recent report described IL-11 as the dominant IL-6 family member during gastrointestinal (GI) tumorigenesis in mice.60 IL-6 antagonists have been approved for the treatment of RA and related diseases but need to be further evaluated in cancer.
Interleukin 1
IL-1 is another major proinflammatory and protumorigenic cytokine produced by a variety of cell types.61 IL-1 induces fever and plays an important role in sepsis. There are two isoforms of IL-1: IL-1α and IL-1β, both of which activate NF-κB, JNK, p38, and ERK via IL-1 receptor (IL-1R) and can induce expression of other cytokines. IL-1α and IL-1β have similar functions although they are only 26% similar at the protein level. Several IL-1 antagonists have been developed and are used for the treatment of auto-inflammatory conditions. These inhibitors also need to be tested for efficacy in cancer.
Interleukin 17A
IL-17A is a member of the IL-17 family and is mainly produced by IL-17-producing T-helper (Th17) cells, γδ T cells, and innate lymphoid cells (ILCs).62, 63 IL-17A normally provides protection against extracellular bacteria and fungi and is associated with autoimmune diseases, such as psoriasis. IL-17A activates NF-κB, JNK, p38, and ERK via IL-17 receptor A (IL-17RA) in many cell types and promotes tumorigenesis, metastasis, angiogenesis, and chemotherapy resistance.64 IL-17A induces
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree