Nina Singh, Ajit P. Limaye
Infections in Solid-Organ Transplant Recipients
Advances in surgical techniques and immunosuppressive regimens have been pivotal factors in optimizing outcomes after transplantation. The introduction of cyclosporine in the 1980s and tacrolimus a decade later heralded the era of modern immunosuppression, and transplantation advanced from being a quasi-experimental procedure to an established and accepted modality of treatment for a variety of end-organ diseases. Table 313-1 depicts the most recent data on graft and patient survival released by the United Network for Organ Sharing (UNOS).1 The best results are for living-related kidney transplantation; approximately 99% of the recipients are alive and 96% have a functioning allograft 1 year after transplantation. The greatest progress may have been in liver transplantation, in which the 1-year survival rate before the use of cyclosporine was only 32% compared with the current survival rate of 89%. The survival rates for lung, heart-lung, and intestinal transplant recipients have lagged behind the other groups, but these procedures were only introduced in the 1980s, and experience has been gained slowly because of the limited number of operations performed. Currently, fewer than 150 intestinal transplants are performed annually in the United States; however, 1-year patient survival rates at high-volume experienced centers currently approach 90%.
TABLE 313-1
Overall Graft and Patient Survival Rates by Organ Transplant
| TYPE OF TRANSPLANT | NUMBER OF TRANSPLANTS | GRAFT SURVIVAL (%) (1 YEAR) | PATIENT SURVIVAL (%) (1 YEAR) |
| Kidney (living donor) | 5770 | 96.5 | 98.7 |
| Kidney (deceased donor) | 11,043 | 91.4 | 95.9 |
| Kidney-pancreas | 795 | 86.2/93.2* | 96.6 |
| Heart | 2322 | 88.4 | 88.9 |
| Liver (deceased donor) | 6392 | 85.5 | 89.3 |
| Intestine | 129 | 74.8 | 78.4 |
| Lung | 1822 | 80.0 | 82.3 |
| Heart-lung | 27 | 89.8 | 90.3 |
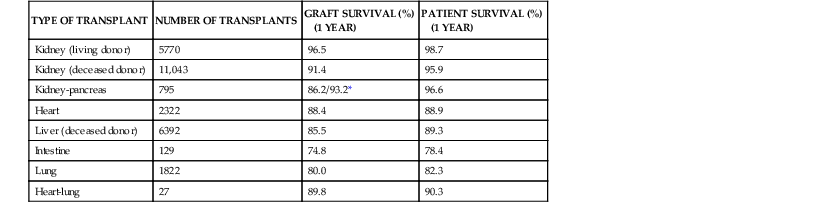
* In the kidney-pancreas recipients, pancreas and kidney graft survival rates are 86.2% and 93.2%, respectively.
Modified from the 2009 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 2007. Rockville, MD: Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2009.
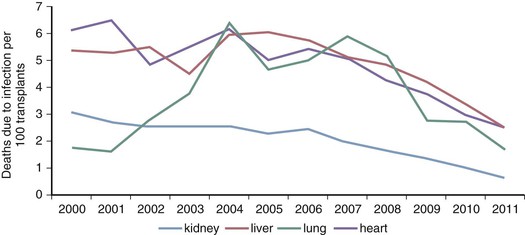
Improvements in graft and patient survival have been paralleled by a decline in mortality from infections (Fig. 313-1). The most recent data from the Organ Procurement and Transplant Network show that infection-related mortality within the past decade has continued to decline in all types of organ transplants (see Fig. 313-1). Nevertheless, infections still account for 13% to 16% of the deaths in kidney and heart transplant recipients and up to 21% in lung transplant recipients (Table 313-2). In liver transplant recipients, approximately 50% of the mortality within the first post-transplant year was infection associated.2
TABLE 313-2
Leading Causes of Death in Organ Transplant Recipients 10 Years after Transplantation
| TYPE OF TRANSPLANT | PERCENTAGE OF DEATHS DUE TO: | ||||
| Vascular Events* | Infection | Malignancy | Graft Failure | Other | |
| Kidney | 22.4 | 15.4 | 8 | — | — |
| Liver | 12.5 | 15.5 | 11.8 | — | 11.3† |
| Heart | 20 | 13 | 7.4 | 16.7 | 9.3† |
| Lung | 9.4 | 21.4 | — | 20.5 | 18.4† |
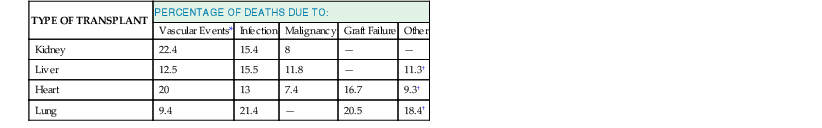
* These include cardiovascular and cerebrovascular events.
† Other causes of death are multiorgan failure in liver and heart transplant recipients and pulmonary diseases (acute respiratory distress syndrome, bronchiolitis obliterans, embolism, and pulmonary hypertension) in lung transplant recipients.
Data from Organ Procurement and Transplantation Network database as of November 9, 2012.
Although calcineurin inhibitors have been the mainstay of immunosuppression for more than 3 decades, long-term outcomes remain suboptimal, largely due to renal dysfunction and metabolic complications from cumulative exposure to these agents. For example, chronic renal dysfunction develops in 7% to 21% of the organ transplant recipients and increases the risk of death by approximately fourfold.3 These concerns have spawned a growing interest in new regimens that enhance immunologic tolerance (e.g., interleukin-2 [IL-2] receptor inhibitors [basiliximab] and T cell–depleting antibodies [alemtuzumab or thymoglobulin]). T cell–depleting agents increase the risk of cytomegalovirus (CMV) infection. Use of these agents as antirejection but not induction therapy also confers a higher risk of fungal infections.
Time of Occurrence of Infections after Transplantation
The frequency, types of infections, and specific pathogens encountered after transplantation generally follow a predictable time to onset. Thus, infections in transplant recipients must be evaluated in the context of time since transplant. Evolving medical practices and preventive strategies have modified the risk and timeline of many infections as discussed later.
Infections during the First 30 Days
Infections during this period are primarily surgical or technical complications related to transplantation or are health care associated. Bacterial infections, including those due to antimicrobial-resistant pathogens, are by far the most frequently occurring infections; vascular-catheter infections, health care–associated pneumonia, Clostridium difficile colitis, and surgical-site infections are the most common types. In the absence of antifungal prophylaxis, candidiasis and aspergillosis typically occur in the first month after transplantation. Except for the reactivation of herpes simplex virus (HSV), viral infections are not commonly encountered very early after transplant. Certain donor-transmitted infections (discussed later) may also manifest during this period.
Infections between 30 and 180 Days
Although nosocomial infections continue to pose a threat in patients requiring prolonged hospitalization, surgical infections decline in importance after 1 month and typical opportunistic infections associated with the immunosuppressed state emerge. These include Pneumocystis jirovecii, fungi, Toxoplasma gondii, Nocardia, and most importantly, CMV. Historically, CMV infection and disease have occurred between 4 and 6 weeks after transplant. However, in the era of routine use of antiviral prophylaxis (typically given for 3 to 6 months after transplant), most CMV disease now occurs later, after antiviral prophylaxis has been discontinued (“late-onset” CMV disease). Pneumocystis pneumonia and, to a large extent, toxoplasmosis are seen infrequently today because of the effectiveness of trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis. Invasive aspergillosis has long been regarded as an early-occurring infection. However, most cases now occur between 1 and 6 months after transplant. Notably, in lung transplant recipients Aspergillus infections now occur after 12 months; the median time to onset after transplant was 357 days in one report4 and 33 ± 19.6 months in another.5 Widespread use of mold-active antifungal prophylaxis and chronic rejection or its treatment, or both, likely account for these trends.
Infections Occurring 6 Months or Later
Infections more than 6 months after transplantation have been mostly community acquired and similar to those seen in the general population. However, contemporary data from the Spanish Consortium for the study of infections in transplant recipients show that although the rate of infections declined from 3.5/1000 transplant-days in the first 6 months to 0.4/1000 transplant-days in the late period, the etiology of infections and infection-related mortality was similar in the two periods.6 Patients with chronic graft dysfunction and graft-related reoperations are at higher risk for late infections. Heart, kidney, and liver transplant recipients have lower rates (≈0.3/1000 transplant-days), whereas pancreas and lung transplant recipients have the highest incidence rates of late infections (0.76 and 1.4/1000 transplant-days, respectively).6
Other late manifestations may represent reactivated or chronic viral infections. Herpes zoster may occur at any time after transplantation. A number of tumors related to viral infection may also occur late, with warts (verruca vulgaris) the most frequent infection. Also, some lymphomas and lymphoproliferative syndromes related to Epstein-Barr virus (EBV) occur after 1 year. Finally, although the risk for classic opportunistic infections declines, it never disappears completely. Infections due to mycoses such as cryptococcosis or histoplasmosis often manifest late and without an apparent inciting event or change in immunosuppression.
Types of Transplants and Characteristics of Infections
Table 313-3 presents the type, severity, and characteristic sites of infections in kidney, heart, heart-lung, and liver transplant recipients observed during the first year after transplantation. Kidney transplant recipients have the lowest number of episodes of infection per patient, whereas liver and heart transplant recipients have intermediate rates. Heart-lung or lung recipients, by contrast, have more than three times the number of infections. The liver transplant group has almost twice the rate of bacteremia of the renal group. Invasive fungal infections are frequent in liver and heart-lung or lung recipients, intermediate in heart recipients, and rare in renal recipients. Table 313-3 also shows that the most common sites of infection are closely related to the site of surgery.
TABLE 313-3
Frequency, Types of Infections, and Sources of Infections Occurring in the First Year after Transplantation
| TYPE OF TRANSPLANT | INFECTION EPISODES PER PATIENT | BACTEREMIA (%) | CMV DISEASE* (%) | FUNGAL INFECTIONS (%) | MOST COMMON SOURCE |
| Kidney | 0.98 | 5-10 | 8 | 0.7 | Urinary tract |
| Heart | 1.36 | 8-11 | 25 | 8 | Lung |
| Heart-lung | 3.19 | 8-25 | 39 | 23 | Lung |
| Liver | 1.86 | 10-23 | 29 | 16 | Abdomen and biliary tract |
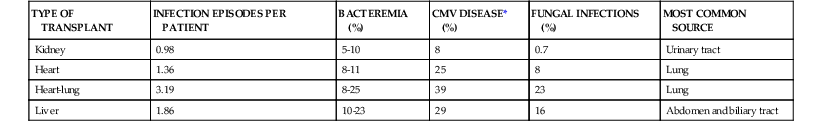
* Numbers reflect CMV disease rates in the absence of routine antiviral prophylaxis.
CMV, cytomegalovirus.
Kidney Transplant Recipients
Most infections, including bacteremia in these patients, arise from the urinary tract. By 3 years after transplantation, more than half of all renal recipients will have been diagnosed with upper or lower urinary tract infection.7 Many infections are uncomplicated cystitis, but graft pyelonephritis occurred in 13% of 1387 renal recipients over a 13-year period.8 In this study, pyelonephritis occurring in the first 3 months was associated with reduced graft survival, but later infections were not. However, a larger, multivariate analysis of more than 28,000 kidney recipients in the Medicare database showed that urinary tract infection after 6 months was associated with worse long-term graft function and survival.7 Abnormalities such as ureteral reflux, strictures at the ureterovesical junction, or neurogenic bladder should be sought in patients with recurrent infections. Administration of an extended course of antibiotics (≥4 weeks) and consideration of secondary antibiotic prophylaxis is reasonable in patients who have severe graft pyelonephritis or recurrent infections. Asymptomatic bacteriuria is common in kidney transplant recipients, and there are insufficient data for or against routine screening and treatment in this setting.
The clinician should also be alert to the occurrence of uncommon urinary pathogens. For example, urinary tract tuberculosis may arise from a focus in the native kidney. Mycoplasma hominis may cause a breakdown of the ureterovesical anastomosis with subsequent graft loss. Histoplasmosis may involve the transplanted kidney and cause renal failure. Adenovirus may cause hemorrhagic cystitis or nephritis, or both. BK virus is an important cause of renal allograft infection and is not typically associated with clinical signs or symptoms.
Historically, pneumonia occurred in 25% to 30% of kidney transplant recipients and was the most common infectious cause of death. In the past, transplantation-associated pneumonia was often due to CMV and opportunistic infections such as fungi, Nocardia, and Pneumocystis. More recently, these opportunistic agents have come under better control, and conventional bacterial pathogens have become relatively more common in transplant populations. Wound infections are infrequent (4% to 5%) but may be a serious problem, particularly if they involve the perinephric space. Body mass index greater than or equal to 30 kg/m2, urinary leak, reoperation through the transplant incision, mycophenolate mofetil use, and diabetes are risk factors for infection.
Some renal transplant recipients continue to have frequent problems with infection even after the first 6 months. These patients have often received excessive immunosuppression or have chronic dysfunction of the allograft or other major organs. Some suffer from chronic viral infections such as hepatitis C virus (HCV).
Heart Transplant Recipients
The most common infections after heart transplantation are bacterial pneumonias, urinary infections, herpes virus infections, and invasive fungal infections. Pneumonia in heart transplant recipients is mostly caused by common pathogenic bacteria (Table 313-4). Although the incidence of pneumonia is highest during the first few months after transplantation, bacterial pneumonias occur sporadically in the late post-transplantation period, after the patient has recovered from the immediate effects of surgery. In a survey of 620 heart transplant recipients at Stanford University, infections were the most common cause of death.9
TABLE 313-4
Microbial Causes of Pneumonia in Transplant Recipients
Early Pneumonia (≤30 Days)
Common Causes
Gram-negative bacilli
Staphylococcus aureus
Aspiration
Less Common Causes
Aspergillus
Herpes simplex virus
Legionella
Toxoplasma gondii
Late Pneumonia (>30 Days)
Common Causes
Pneumococcus
Hemophilus influenzae
Influenza
No cause identified
Less Common Causes
Pneumocystis
Nocardia
Legionella
Aspergillus
Gram-negative bacilli
Staphylococcus aureus
Aspiration
Cytomegalovirus
Varicella zoster virus
Paramyxoviruses
Tuberculosis
Coccidioidomycosis
Histoplasmosis
Cryptococcus
Respiratory viruses*
* These include parainfluenza, respiratory syncytial virus, metapneumovirus, coronavirus, rhinovirus, and adenovirus.
Mediastinitis and sternal wound infections are postoperative complications unique to heart and heart-lung transplant recipients and occur in approximately 2.5% of patients. The pathogens seen are similar to those observed in other patients undergoing cardiothoracic surgery, with staphylococci predominating. One must also be alert to the possibility of unusual pathogens. Mediastinitis and sternal wound infections in heart recipients have been caused by M. hominis, Legionella, Aspergillus, mucormycetes, and Nocardia. Factors that are thought to predispose to mediastinitis in this population are repeat operations for hemorrhage, use of antirejection therapy, and diabetes mellitus. Surgical drainage is a crucial component of treatment of mediastinitis in the transplant patient.
Left-ventricular assist devices (LVADs) are now widely used as a bridge to transplantation. Infections of these devices are common and fall into distinct types: driveline infections, which are often limited to the exit site; deep infections in the pocket surrounding the device; and internal infection of the device, which is often associated with bloodstream infection. Management of these infections is challenging; however, in many cases, the infection can be controlled well enough to permit transplantation. Available experience suggests that the use of antimicrobial therapy before, during, and after transplantation is associated with fewer relapses than short-course therapy. Although pretransplant LVAD infection is associated with a higher rate of peri–heart transplant complications, long-term outcomes appear to be reasonable and LVAD infection is not considered a contraindication to heart transplant.10
A number of other infections occur more commonly in heart recipients than in patients receiving other types of transplants. These include toxoplasmosis, nocardiosis, and Chagas’ disease. Toxoplasma-seronegative heart recipients are at risk for toxoplasmosis because the infection can be acquired from organisms encysted in the heart muscle of toxoplasma-seropositive donors. Clinical toxoplasmosis usually occurs a few weeks to a few months after transplantation and is manifested by necrotizing pneumonitis, myocarditis, and encephalitis. Before the use of preventive therapy, the rates of toxoplasmosis were 60% to 80% in donor toxoplasma seropositive/recipient seronegative patients and approximately 5% to 10% in toxoplasma seropositive recipients. Clinical toxoplasmosis is now uncommon in heart recipients, likely because of the use of TMP-SMX for Pneumocystis prophylaxis.9
Symptomatic reactivation of Trypanosoma cruzi may be seen in immunocompromised patients, including transplant recipients. About one fourth of patients who undergo heart transplantation for cardiomyopathy caused by chronic T. cruzi infection have relapses of acute Chagas’ disease, with clinical manifestations of fever, myocarditis, and skin lesions. The disease can usually be controlled with chemotherapy. Detection of parasitemia by microscopy or polymerase chain reaction (PCR) in blood samples is useful to monitor for reactivation and guide chemotherapy after transplantation.
Nocardia infections have also been more frequently reported in heart and lung transplant recipients than in recipients of kidney or liver transplants, but the biologic reason for this increased rate of nocardiosis is unknown. The doses of TMP-SMX used for Pneumocystis prophylaxis might not provide reliable protection against Nocardia infection.
Heart recipients frequently suffer trauma to the tricuspid valve and right ventricular endocardium from repeated endomyocardial biopsies, a common post-transplantation practice. Heart transplant patients with cardiac valvulopathy are at high risk for adverse outcomes from endocarditis, and prophylaxis with dental procedures is considered to be appropriate in this group.11 Most cases occur during the first year after transplantation and may be caused by unusual organisms such as Aspergillus or Legionella species.
Lung and Heart-Lung Transplant Recipients
Lung and heart-lung transplant recipients have infectious problems largely similar to those of heart transplant recipients, but the infections are more frequent and severe. The heightened vulnerability of the transplanted lung to infection is multifactorial. In addition to mechanical factors related to decreased mucociliary clearance, diminished lymphatic drainage and ablation of the cough reflex appear to play an important, if poorly understood, role. The types of infections seen in lung transplant recipients are similar to those in heart-lung recipients, although the overall survival rate is better. Heart-lung transplantation is now usually reserved for patients who have Eisenmenger’s syndrome and whose cardiac abnormalities cannot be surgically repaired. Single- and double-lung procedures leave the donor heart available for another patient with end-stage heart disease. A unique aspect of single-lung transplantation is the occurrence of infections in the native lung that may be predisposed to infection because of defects of ventilation or perfusion caused by the underlying lung disease.
Lung and heart-lung transplant recipients experience a high rate of bacterial lung infections, especially during the first few weeks after transplantation. These patients also have higher rates of mediastinitis, invasive fungal infections, and CMV pneumonia than heart recipients. Some patients undergoing lung transplantation are chronically colonized with multidrug-resistant bacteria (e.g., cystic fibrosis, non–cystic fibrosis bronchiectasis) and are therefore at high risk for postoperative infection at the time of transplant surgery. Antimicrobial treatment directed against pretransplant colonizing bacteria is typically given for several weeks after transplant. Pretransplant colonization with certain Burkholderia spp. (Burkholderia cenocepacia in particular) has been associated with severe infection and decreased survival after transplant, and thus many transplant centers consider pretransplant B. cenocepacia colonization or infection to be a contraindication to lung transplantation.
Heart-lung and lung transplant recipients develop invasive pulmonary aspergillosis more frequently than patients with other types of organ transplants.12 Risk factors have included pretransplant colonization, single lung transplant, and augmented immunosuppression for allograft rejection. A unique form of aspergillosis involving the airway mucosa or bronchial anastomotic sites, called tracheobronchial aspergillosis, is observed almost exclusively in lung and heart-lung recipients. The airway lesions of this disease can be directly visualized during bronchoscopy. This form of aspergillosis has a better prognosis than aspergillosis invading the lung parenchyma. The predisposition of lung recipients to invasive aspergillosis has led many lung transplant centers to routinely use various targeted antifungal preventive strategies. Oral azoles and inhaled amphotericin are the most widely used agents.
In the late post-transplant period, up to two thirds of patients eventually develop obliterative bronchiolitis. This process is the main pathologic manifestation of chronic rejection of the lung. It has been associated with recurrent pulmonary infections and is one of the major causes of death in lung transplant patients. The lung allograft is also particularly susceptible to viral infection, as has been documented for CMV, HSV, paramyxoviruses, and adenovirus infections. Accordingly, lung transplant centers often utilize aggressive regimens for prevention of herpesvirus infections and include diagnostic tests for respiratory viruses in the workup of patients who present with acute chest infections. Community-acquired respiratory virus infections (influenza, parainfluenza, respiratory syncytial virus [RSV], metapneumovirus, coronavirus, rhinovirus, and adenovirus) are a significant cause of morbidity and have been associated with both short-term and long-term impairment of allograft infection. Other than for influenza, there are no therapies proven to improve outcomes for these other respiratory viruses.
Pulmonary nontuberculous mycobacterial infections are relatively common in lung transplant recipients and may arise from pretransplant colonizing strains or exogenous acquisition. Mycobacterium abscessus has been associated with severe infection after transplant, and some centers exclude patients with pretransplant M. abscessus infection (colonization) or disease.13 Pretransplant colonization with other nontuberculous mycobacteria does not appear to be as closely associated with decreased survival or severe post-transplant infection, or both.
Prospective donors for lung transplantation are intubated in intensive care units. Therefore, the airways of these donors are often colonized with microorganisms, and occult parenchymal infection may be present. Before implantation of the lungs, it is customary to obtain cultures and Gram stains of the donor airways to guide antibiotic therapy in the recipient. Initial antibiotic prophylaxis should be aimed at common nosocomial pathogens encountered in the intensive care unit, including methicillin-resistant Staphylococcus aureus and enteric gram-negative bacilli. Another problem unique to lung transplantation has been dehiscence of the airway anastomosis. This occurs during the first few weeks after transplantation. It is frequently associated with bacterial or fungal infection at the anastomotic site. The incidence of this complication appears to be declining.
Liver Transplant Recipients
Liver transplant recipients have higher rates of infection than renal or heart transplant recipients, and most deaths are associated with infection, either as a primary or as a secondary cause (see Table 313-2). Bacterial infections occur in 59% to 68% of the patients after liver transplantation. About half of these infections occur within 2 weeks after transplant. The most important sites of infection are the abdomen and biliary tract, surgical wound, lungs, and bloodstream, with or without associated catheter infection.
Liver transplant recipients have a high rate of fungal infections. The incidence has ranged from 15% to 42%, with a case-fatality rate of 25% to 82%.14,15 Risk factors for invasive fungal infections include renal dysfunction, fulminant hepatic failure, longer operative time, retransplantation, hepatic iron overload, and colonization with Candida at the time of transplantation.16
Candida is the predominant pathogen, causing 62% to 88% of the invasive fungal infections in this population, although it has declined in relative importance compared with Aspergillus, other molds, and Cryptococcus. Almost one third of candidal infections in liver transplant recipients are caused by non-albicans species. Fluconazole prophylaxis was found to be a risk factor for the emergence of non-albicans Candida species as pathogens in liver transplant recipients. Invasive candidiasis contributes significantly to mortality after liver transplantation. In a case-controlled study, liver transplant recipients with invasive candidiasis had a 36% mortality rate, compared with 2% in the control patients.
Invasive aspergillosis occurs in 1% to 8% of liver transplant recipients. Major risk factors for invasive aspergillosis in liver transplantation include retransplantation, fulminant hepatic failure as an indication for transplantation, renal dysfunction and hemodialysis, and poor allograft function.12 Most Aspergillus infections now occur more than 90 days after transplantation, a possible consequence of improved early management and delayed occurrence of risk factors such as CMV infection. Liver transplant recipients are uniquely predisposed to disseminated aspergillosis.
Abdominal Infections in Liver Transplant Recipients
Transplantation of the liver differs from other transplant operations in the length and difficulty of the surgery and the frequency of bleeding. In addition, many liver transplant recipients have poor nutrition and severe metabolic difficulties. Abdominal infections after liver transplantation are often related to technical aspects and complications of the operation. For example, anastomosis of the biliary duct to a Roux-en-Y loop of jejunum is associated with more intra-abdominal infections, especially invasive fungal infections, than is primary anastomosis of the donor’s to the recipient’s common bile duct.
Most liver abscesses in the transplanted liver are related to surgical problems such as biliary stricture and hepatic artery thrombosis. The organisms responsible are gram-negative enteric bacilli, enterococci, and anaerobes. The diagnosis is made by ultrasonography or computed tomography. Treatment with drainage and intravenous antibiotics is usually successful, provided the source is biliary infection and any structural abnormalities can be corrected. If hepatic artery thrombosis is the predisposing factor, the infectious symptoms can usually be controlled with antibiotics, but retransplantation may be necessary.
Cholangitis after liver transplantation also results from technical problems. The most common predisposing problem is biliary stricture. Patients with strictures may have periodic bouts of cholangitis. Some patients improve after dilatation procedures or stent placement, but in others operative repair is necessary. It may not be easy to make a firm diagnosis of cholangitis because many patients do not manifest the classic Charcot’s triad of fever, abdominal pain, and jaundice. The clinical presentation may resemble allograft rejection. The diagnosis is more reliable if bacteremia is present or if a liver biopsy indicates pericholangitis. Empirical treatment for cholangitis should include antibiotics to cover gram-negative enteric bacilli, enterococci, and anaerobes. Procedures such as T-tube cholangiography and endoscopic retrograde cholangiopancreatography may be followed by cholangitis and, occasionally, by bacteremia. Therefore, a single dose of a prophylactic antibiotic is recommended.
Peritonitis can accompany other intra-abdominal infections and frequently complicates biliary leaks or disruption of an abdominal viscus. Bile peritonitis may occur after extraction of a T-tube. It is often well tolerated and may resolve by itself, but occasionally the leak persists and the chemical peritonitis becomes secondarily infected. The most common organisms involved in peritonitis are enterococci and aerobic enteric gram-negative rods, but staphylococcal and candidal infections are not infrequent. Treatment of established peritonitis requires antibiotic therapy, together with drainage of associated abscesses and repair of technical problems such as biliary leaks.
Abdominal abscesses are usually found in patients who have had frequent or lengthy abdominal operations. Nearly one third of the abdominal abscesses are associated with bacteremia. The location is frequently subhepatic, but splenic, pericolic, and pelvic abscesses are also seen. Most patients with abscesses have undergone an abdominal operation within the preceding 30 days. Although common enteric organisms cause most abscesses, staphylococci and Candida are also seen. Imaging studies usually define the location of the abscess. As with any other abscesses, the appropriate treatment is a combination of drainage and antibiotics directed against the responsible pathogens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree