Susan E. Beekmann, David K. Henderson *
Infections Caused by Percutaneous Intravascular Devices
The relentless progress of medical science and technology has been accompanied by the development of a host of new diagnostic and therapeutic medical devices, each of which is associated with its own complications. Included in the list of devices and the complications of their use to be discussed in this chapter are peripheral and central intravenous catheters, including nontunneled central catheters and tunneled (Hickman or Broviac) catheters with or without the Groshong tip (which remains closed unless the catheter is in use), peripherally inserted central venous catheters (PICCs), totally implanted intravascular access devices (ports), pulmonary artery catheters, and arterial lines.
As early as 1977, Maki1 suggested that more than 25,000 patients develop device-related bacteremia in the United States each year. The burgeoning use of an ever-expanding array of vascular access devices in medicine has resulted in even more complications associated with their use. Rates of bacteremia associated with the use of intravascular devices increased significantly into the early 2000s. More recent data indicate that the rates of CLABSIs are decreasing, perhaps as a result of prevention programs implemented in many hospitals since 2001. In 2009, the Centers for Disease Control and Prevention (CDC) has estimated that approximately 18,000 central line–associated bloodstream infections (CLABSIs) currently occur in intensive care units (ICUs) each year, compared with 43,000 in 2001.2 An intense focus on prevention of health care–associated infections, including CLABSIs, and requirements for the incorporation of performance measures into regulatory and financial reimbursement systems has the potential to significantly reduce these infections.3,4
Such device-associated infections occur as sporadic cases as well as in case clusters caused by the same organism. Vascular catheters have become an increasingly important source of bacteremias, increasing from 3% in the mid-1970s to 19% in the early 1990s.5 Primary bacteremias (i.e., no apparent local infection elsewhere caused by the same organism), including intravascular catheter sources, account for approximately one half of all ICU-related bacteremias.6,7 In cancer patients, 56% of all bloodstream infections from 1999 to 2000 were CLABSIs.8 The problem of iatrogenic, device-associated bacteremia is not unique to the United States; in one prospective study of bacteremia from Australia, nosocomial bacteremias accounted for 40% of all cases of bacteremia, and half of the nosocomial cases were device associated.9 Although most data on CLABSI rates are derived from studies in ICUs, Marschall and colleagues10 reported that the incidence of CLABSIs in non-ICU, general medical patients was comparable to the rate in ICU patients.
Both local and systemic infection may result from contamination of intravascular devices. Local cellulitis, abscess formation, septic thrombophlebitis, device-associated bacteremia, and endocarditis all occur as complications of intravascular therapy and monitoring.
Pathogenesis
For intravascular device–related bacteremia to occur, microorganisms must gain access to the extraluminal or intraluminal surface of the device. Microbial adherence and incorporation into biofilms then occurs, resulting first in infection and then, in some instances, in hematogenous dissemination.11 Figure 302-1 illustrates the potential points of access to an intravascular device, each of which has been associated with both sporadic cases and case clusters of nosocomial bacteremia. Whereas the skin entry site has long been thought to be the most important portal of entry for invading microorganisms, the catheter hub–lumen has also been shown to be a major contributor to catheter-related bacteremia.12,13 The most common point of access appears to vary, depending on the duration of time the catheter has been in place. Each of the three major sources of intravenous device–related bacteremia is discussed in the following sections.
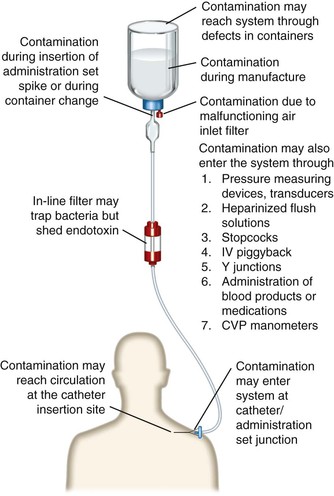
Contamination of the Infusate
Contamination of the fluid administered through the device is a major cause of epidemic intravenous device–related bacteremias. Nonetheless, infusate contamination is a rare cause of bacteremia. Infusion-related sepsis has been reviewed in detail,14 and both manufacture-related15 and in-use16 contamination of infusate have been documented as causes of device-associated sepsis.
Another factor influencing the pathogenesis of infusate-associated infection is the composition of the fluid. Different infusion fluids support the growth of differing pathogens. The microbiology of outbreaks of infusate-related sepsis is somewhat monotonous; pathogens such as Enterobacter, Citrobacter, and Serratia predominate. No infusate is entirely free of risk; even sterile water for injection can support the growth of Burkholderia cepacia.17 Parenteral nutrition solutions are superb substrates for the growth of certain microorganisms.18 Lipid emulsions support bacterial growth extremely well,19 and their use has also been associated with a risk for fungemia caused by the lipid-dependent yeast Malassezia furfur, although not with contaminated infusates.20 This risk has been primarily identified in the neonatal intensive care setting and has been less commonly seen in adults.20 Several additional outbreaks of bacteremias have been linked to compounding pharmacies that adhere to different quality control standards.21,22 One national outbreak of Serratia marcescens bacteremias occurred as a result of contaminated magnesium sulfate solution,22 and two recent outbreaks were associated with contaminated heparin solutions.21,23
Parenteral nutrition solutions may also become contaminated during compounding in the hospital pharmacy.24,25 Two similar outbreaks of Candida parapsilosis infections were linked to the backflow of yeasts into parenteral nutrition solution because vacuum pumps were used improperly.24,25
The composition of the infusate also influences the degree of irritation of the vascular intima at the site of infusion. Fluids that are not isotonic, those at nonphysiologic pH, and those containing particulates all may irritate the vascular wall, thus provoking thrombus formation. Such thrombi may be seeded with microbes—either hematogenously or by direct extension.
Contamination of the Catheter Hub and Lumen (Intraluminal Source)
Currently, bacteremias associated with long-term catheters appear to arise most frequently from an intraluminal source, perhaps after intraluminal colonization with biofilm-producing microbes.26 Contamination of the catheter hub–infusion tubing junction is a significant contributor to device-associated infection.13,27,28 Endemic coagulase-negative staphylococcal bacteremias often arise as a result of contamination of the catheter hub with these organisms. A randomized study examining the effects of a redesigned protective hub found these hubs to be associated with a significantly lower rate of catheter sepsis and culture-positive catheter hubs,29 suggesting that the hub is a common portal of entry for bacteria. Other investigators have incriminated the hub–tubing junction (particularly when it does not allow a good fit) in the pathogenesis of epidemics of coagulase-negative staphylococcal infection.30,31 Maki and Ringer32 found hub contamination to be the second most heavily weighted risk factor for catheter-associated infection in a large, prospective study. Salzman and colleagues33 noted that greater than 50% of episodes of central venous catheter (CVC)-related sepsis occurring in a neonatal ICU were preceded by colonization of the catheter hub with the incriminated organism. In a subsequent experimental study, these investigators found that swabbing the catheter hub with disinfectant substantially reduced the hub’s microbial burden and that preparations containing 70% ethanol were both more effective and more likely to be safer for the patient than preparations containing chlorhexidine.34 Sherertz35 estimated that the hub, lumen, or both contributed two thirds of the microorganisms that infected long-term catheters and that one fourth of the microorganisms were from the skin.
Conversely, some new technologies may be associated with increased risks for catheter-associated infection. Whereas the implementation of needleless intravenous admixture systems provided a safer workplace environment for health care providers, some data suggest that use of these devices may be associated with increased risk for device-associated infection.36,37 Multiple investigations of bacteremia outbreaks associated with needleless devices have suggested that the mechanism for bacteremia may involve contamination from the end cap.38–41 Of interest, different studies have paradoxically found either increased or decreased risk with the same needleless system (e.g., the Interlink device [Baxter, Deerfield, IL]),38,39 leading to the conclusion that the primary risk associated with these devices is related to how the systems are used (e.g., frequency of changing end caps and adherence to recommended infection control procedures) rather than factors intrinsic to the system.39 Appropriate staff education regarding use of these devices and ensuring compliance with the manufacturer’s recommendations is recommended to prevent device-related bacteremia.
Contamination of Skin at the Device Insertion Site (Extraluminal Source)
Many authorities believe that the catheter insertion tract provides the major avenue for the ingress of microbial invaders.* Several studies have focused on microbial colonization around the catheter insertion site as a significant risk factor for catheter-associated infection.44 Supporting this contention are the studies of Cooper and Hopkins that demonstrated organisms on the exterior surface of catheters rather than within the catheter lumen.45 In the prospective study of Maki and Ringer,32 colonization around the catheter insertion site was the most strongly associated risk factor for local catheter infection. Similarly, Safdar and Maki46 determined that most catheter-related bacteremias occurring with short-term noncuffed central catheters were extraluminally acquired and derived from the cutaneous microflora. Skin appears to be the primary source of intravenous device–related bacteremia for short-term catheters placed for an average duration of less than 8 days.47–49
Skin colonization is a dynamic process. Atela and co-workers42 conducted a prospective study to assess the turnover of superficial skin colonization by performing serial quantitative cultures of skin and the catheter hub.4 Strains recovered from the targeted superficial skin sites demonstrated a poor correlation both with strains from previous skin cultures and with catheter tip isolates.42 Herwaldt and colleagues50 examined the source of coagulase-negative staphylococcal bacteremias in hematology-oncology patients and found that the same strain was identified in both skin and blood cultures in only 6 of 20 episodes. The matching strain was isolated only from other sites (primarily nares) in the remaining 70% of episodes, leading these investigators to the conclusion that mucous membranes might be a reservoir for strains of coagulase-negative staphylococci causing bacteremia in immunocompromised patients. Of importance, these investigators were unable to identify colonization with the same strain for the majority of bacteremias; only 4 of the 21 nosocomial bloodstream infections were preceded by colonization with the same strain. Most nosocomial coagulase-negative staphylococcal bacteremias in this study appeared to result from extrinsic introduction of the organism.
Epidemiology
According to the CDC’s National Healthcare Safety Network (NHSN), rates of CLABSIs in 2009 ranged from 1.05 (pooled mean hemodialysis rate) to 1.14 (pooled mean inpatient ward rate) to 1.65 (pooled mean ICU rate) bacteremias per 1000 CVC days.2 These rates reflect a 58% reduction in total estimated CLABSIs from 2001 to 2009.2 The majority of CLABSIs now occur not in ICUs but in inpatient wards and outpatient hemodialysis settings.2 The reduction in incidence of Staphylococcus aureus CLABSIs was greater than for any other pathogen (see later).2
Intravenous device–related bacteremia rates are influenced by patient-related parameters, catheter-related parameters, and hospital-related parameters (Table 302-1). Because of methodologic difficulties in performing appropriate scientific studies to characterize relative risk, many of these risk factors have been identified either retrospectively or in the epidemic setting. Still, each of the patient-related factors identified in Table 302-1 has been associated with an increased risk of device-associated infection.51 Alteration of the patient’s skin microbiota, either as a result of antimicrobial therapy or by colonization with an epidemic strain carried on the hands of hospital personnel, is a common event preceding catheter site infection. Failure of hospital personnel to perform appropriate hand hygiene procedures, particularly in the ICU setting, has been well documented.52–54 Numerous epidemics of device-associated bacteremia have been linked to hospital personnel carrying an epidemic strain on their hands. Manipulating the system for repositioning, for obtaining a sample, or for any other reason increases the likelihood that the catheter may become contaminated.55 The increasing prevalence of multiple drug resistance among health care–associated isolates causing these infections (e.g., vancomycin-resistant enterococci, multiresistant Acinetobacter baumannii, carbapenem-resistant Enterobacteriaceae) has magnified this problem in the past decade.
TABLE 302-1
Risk Factors for Device-Associated Bacteremia
Granulocytopenia
Immunosuppressive chemotherapy
Hematologic malignancy (versus solid tumor)391
Loss of skin integrity (e.g., burns, psoriasis)
Severity of underlying illness
Active infection at other site
Alteration in patient’s cutaneous microflora
Failure of health care provider to wash hands
Contaminated ointment or cream
Catheter composition/construction
Flexibility/stiffness
Thrombogenicity56
Microbial adherence properties and biofilm production60
Size of catheter
Number of catheter lumens65
Catheter function/use
Catheter management strategies—number of entries into the system
Type of catheter (plastic > steel)
Location of catheter (central > peripheral; jugular > femoral > subclavian48,85,392; lower extremity sites > upper extremity sites)
Type of placement (cutdown > percutaneous)
Duration of placement (at least 72 hr > less than 72 hr)32,206*
Emergent placement > elective
Skill of venipuncturist (others > intravenous team)80,206
Type/use of catheter (balloon-tipped, flow-directed > percutaneously placed; central venous > implanted central venous)232
Nursing staffing variables (nurse-to-patient ratio,87 lower regular registered nurse-to-patient ratio, and higher float pool registered nurse-to-patient ratio)88,89,388
* Although several studies support this precept, another has questioned it.
Several catheter characteristics or properties have been suggested to be associated with an increased risk for catheter-associated infection. Catheters that irritate the vascular intima and provoke thrombogenesis and catheters that are made of intrinsically thrombogenic materials are likely to be associated with an increased risk for device-associated infection.56 Older studies suggest that stiff catheters were associated with higher infection rates.57 Such catheters are likely to be more mobile in the insertion tract and are thought to be more thrombogenic. A clear association has been established between the thrombogenicity of a catheter and the risk for device-associated infection.58,59 Despite differences in thrombogenicity, some authorities believe that all catheters become coated with a fibrin sheath soon after placement.60 Currently, the majority of catheters are manufactured with antithrombogenic polymers, such as polyurethane, Teflon, or others.
Catheter composition may influence the risk for infection in another way. Sheth and co-workers61 have shown that certain microorganisms, most notably staphylococci, are able to adhere better to a catheter made from polyvinyl chloride than to a Teflon catheter. Rotrosen and colleagues62 demonstrated increased adherence of Candida spp. to polyvinyl chloride catheters compared with Teflon catheters. In a rabbit model, silicon catheters are easier to infect with S. aureus than are those made of polyurethane, Teflon, or polyvinyl chloride.63 One might hypothesize that materials that facilitate microbial adherence may be associated with an increased risk for device-associated infection. Newer therapeutic interventions have focused on diminishing adherence to the catheter through use of chelating agents, such as ethylenediaminetetraacetic acid (EDTA) or sodium citrate, or ethanol locks.
The physical size of the catheter (and therefore the size of the defect in the skin’s intrinsic host defenses) is also likely to be correlated with increased risk. Similarly, increasing the number of lumens in a catheter has been suggested to increase the risk for catheter-associated infections. Several studies have suggested that the use of multiple-lumen catheters is associated with an increased risk for catheter-associated infection compared with the use of single-lumen catheters39,64,65; although not all studies have found this difference.66,67
The presence of distant infection resulting in hematogenous seeding of the intravascular device has been incriminated in the pathogenesis of device-associated infection in some series.44,68 Several factors may influence the risk for catheter seeding, including catheter composition, local thrombus formation at the catheter insertion site, intensity of bacteremia, the infecting pathogen, duration of catheterization, duration of bacteremia, and the patient’s ability to mount an immunologic response to the infection.
Formation of a bacterial biofilm is now thought to be a virtually universal phenomenon that begins within 3 days after insertion of intravascular devices.60 Biofilm formation is more predominant on the external surface of catheters in place fewer than 10 days, and then predominates intraluminally in catheters in place for at least 30 days.69 Microorganisms embed themselves in and under the biofilm layer and become the source of intraluminal colonization and, eventually, the sources of CLABSIs.69 Certain chelators, including EDTA and sodium citrate, inhibit bacterial growth and fibrin formation.70 Ethanol lock therapy, in which a high concentration of ethanol is instilled within a catheter and left to dwell, also results in biofilm reduction or eradication.69 The presence of fibrin deposits may explain the difficulty in treating totally implanted venous access port bacteremias without removal of the device.71,72 Antibiotic concentrations should be 100 to 1000 times greater to kill sessile bacteria within a biofilm than for planktonic bacteria.73–76
Finally, the manner in which the catheter is used may influence risk. For example, risks for infection with pulmonary artery catheters may be higher because of the manner in which they are used.77 In critically ill patients, these catheters are used intensively (although now somewhat less frequently than in the recent past): they are frequently repositioned to obtain accurate readings, they are used to obtain samples for the measurement of cardiac output, and they can be used to obtain mixed venous blood to measure oxygen and carbon dioxide tensions.
Catheter management, including both insertion and maintenance, also may influence risk for infection. Several studies have shown that catheters placed by less experienced personnel are at increased risk for infection.78,79 Another study analyzed the efficacy of using a skilled team for the placement of peripheral intravenous catheters.80 In this study, an intravenous therapy team significantly reduced both local and bacteremic complications associated with the placement of peripheral intravenous catheters, in part related to the timely replacement of the catheters. Two studies suggest that insertion of CVCs with less than maximal sterile barriers increases the risk of catheter-related infection.48,81 Several studies have suggested that the number of times the system is entered also influences the risk for infection.55,82,83 More than a single attempt to insert the catheter has also been found to be a risk factor for bacteremia,67 as has the number of venous catheters in place and the intensity of their use.84 Insertion at a subclavian rather than a femoral site is clearly associated with a lesser risk of both infectious and thrombotic complications.65,85,86 In addition to the factors outlined previously, the risk of developing catheter-associated bacteremia is related to the patient and his or her intrinsic host defense mechanisms, as well as to factors related to the patient’s hospital environment or therapy (see Table 302-1). The physician cannot alter most such patient-related factors; however, these data can be used when evaluating the risks associated with, the necessity for, and the duration of intravenous therapy.
In addition to patient-related risk factors, several hospital-related risk factors for CLABSIs have been either identified or proposed (see Table 302-1). In contradistinction to the patient-related factors, such hospital-related factors can often be altered for patient benefit. Nurse staffing variables, including nurse-to-patient ratio, level of training, and permanent assignment to the unit (“float” nurse vs. regular unit staff nurse), have been shown to affect bacteremia rates.87–89 As noted previously, the level of experience of the individual inserting the catheter (i.e., the number of previous catheter insertions) was also found to affect catheter-related bacteremia rates.90 In addition, a number of studies have found that an education program focusing on risk factors and practice modifications is associated with decreased rates of CLABSIs.91,92
Microbiology
Staphylococci continue to predominate as the most frequently encountered pathogens in device-related infections. Although S. aureus is a frequent cause of device-associated infection, the coagulase-negative staphylococci are the most common causes of these infections, especially in immunocompromised patients and those in whom long-term central venous access is required.93 Multiple reports indicate that CLABSIs caused by S. aureus have decreased more than other pathogens over the last decade,2,94
Although there are some minor microbiologic differences among the devices or therapies under discussion, as a genus, Staphylococcus accounts for the majority of the episodes of bacteremia associated with these devices.5,95 Studies suggest that coagulase-negative staphylococci may be able to adhere to plastic catheters more aggressively than can other organisms.96 This property would result in a selective advantage for coagulase-negative staphylococci in causing device-associated infections.
Other commonly encountered isolates are listed in Table 302-2. One study of patients with hematologic malignancies and having Hickman catheters inserted identified a predominance of gram-negative organisms (68%) causing catheter-related bacteremias in this non-neutropenic population.97 Raad and colleagues8 reported that gram-negative bloodstream infections in cancer patients with solid tumors were most likely catheter related. Our own experience would suggest that gram-negative bacilli may predominate among stem cell transplant recipients, particularly those who have gastrointestinal involvement with graft-versus-host disease. The past decade has witnessed an increasing occurrence of CLABSIs caused by multiresistant gram-negative rods, most notably A. baumannii.98 CLABSIs caused by A. baumannii often occur in critically ill, immunosuppressed, highly antimicrobial agent–experienced patients who can ill afford any bacteremia, let alone one caused by multidrug-resistant bacteria.99
TABLE 302-2
Microbiology of Device-Associated Bacteremia

* Most common pathogen for long-term lines; also associated with lipid infusions in neonates.
† Frequently associated with contaminated infusate.
‡ Most often associated with total parenteral nutrition; usually along the catheter path but occasionally as a result of contaminated infusate.
§ May arise from a water source (e.g., infusate) or may reflect cutaneous colonization.
‖ C. jeikeium bacteremia occurs almost exclusively in severely immunosuppressed patients who are or have been receiving broad-spectrum antibiotics and who have indwelling intravascular devices.
¶ A. baumannii (often multiply drug-resistant) is becoming increasingly prevalent as a pathogen in intensive care units, especially among critically ill patients who require life support interventions (e.g., ventilator support) and those who have received multiple courses of antimicrobials.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








