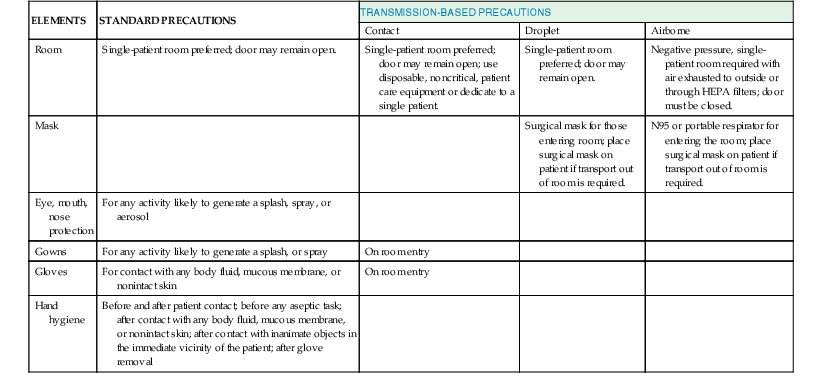
Michael B. Edmond, Richard P. Wenzel Keywords airborne precautions; antibiotic stewardship; contact precautions; droplet precautions; hand hygiene; health care–associated infections; health care epidemiologist; health care epidemiology; infection control; infection prevention; infection preventionist; isolation precautions; nosocomial infections Infection control as a formal discipline in the United States developed during the late 1950s, primarily to address the problem of nosocomial staphylococcal infections. Over the next 50 years, the field of infection control developed slowly, initially focused on surveillance for health care–associated infections (HAIs), then incorporating the science of epidemiology to elucidate risk factors for HAIs. However, three pivotal events signaled the beginning of a new era in health care epidemiology—the Institute of Medicine’s 1999 report on errors in health care, which included HAIs1; the 2002 Chicago Tribune exposé on HAIs,2 which was the beginning of the mainstream media’s interest in this topic; and the publication in 2004 and 2006 of dramatic reductions in bloodstream infection rates by simply standardizing the process of central venous catheter insertion.3,4 This new era in health care epidemiology is characterized by consumer demands for more transparency and accountability, increasing scrutiny and regulation, and expectations for rapid reductions in HAI rates.5 The paradigm shifted from viewing most HAIs as an unpreventable “cost of business” to the vast majority being preventable. Accordingly, the focus for hospital programs shifted from infection control to infection prevention, which required rapid identification of infections and timely actions to analyze them, as well as playing an active role in the implementation of interventions for infection reduction. The primary role of an infection prevention program is to reduce the risk for hospital-acquired infection, thereby protecting patients, employees, health sciences students, volunteers, and visitors. HAIs develop in 1.7 million patients yearly in the United States, accounting for approximately 100,000 deaths,6 at a direct cost of $37 to $45 billion.7 However, these estimates are now 10 years old, and given the intense efforts under way since then, it is highly likely that significant reductions have occurred. The functions of an infection prevention program vary from institution to institution but can generally be divided into the following areas: (1) surveillance, (2) isolation of patients with transmissible pathogens, (3) outbreak investigation and management, (4) education, (5) employee health, (6) the monitoring and management of institutional antimicrobial use and antibiotic resistance, (7) the development of infection prevention policies and interventions, (8) environmental hygiene, and (9) new product evaluation. In some hospitals, quality improvement and patient safety are also undertaken through the hospital epidemiology program. In the academic setting, additional functions of the program may include research and the provision of consultative services to other acute-care and long-term care facilities, public health agencies, and the university campus. The major functions of the effective hospital epidemiology program are listed in Table 300-1, and some of them are discussed in further detail here. TABLE 300-1 Functions That May Be Served By Infection Prevention Programs The first aim of surveillance is to determine endemic rates of infection. Once these rates have been established, an outbreak can be identified when its rate of occurrence is significantly higher than the endemic rate. The importance of surveillance was demonstrated nearly 3 decades ago by the Study on the Efficacy of Nosocomial Infection Control, which found a 32% reduction in HAIs in hospitals with active surveillance programs compared with hospitals without such programs.8 Data from hospitals in the National Nosocomial Infection Surveillance System demonstrated that from 1990 to 1999, nosocomial bloodstream infections decreased by 44% in medical intensive care units (ICUs), 32% in pediatric ICUs, and 31% in surgical ICUs.9 As hospitals gained experience in standardization of patient care processes (e.g., central venous catheter insertion, head of bed elevation), further reductions in HAIs have been observed. The Centers for Disease Control and Prevention (CDC) recently reported that in the time period 2008 through 2011 there was a 41% reduction in central line–associated bloodstream infections and a 17% reduction in surgical-site infections, with only a 7% reduction in catheter-associated urinary tract infections in the time period 2009 through 2011.10 Over the past several years, many hospitals have begun to monitor compliance with process measures, because feedback to health care workers on compliance with best practices more forcefully drives compliance than simply providing feedback on infection rates.11,12 Surveillance for HAIs has generally targeted areas of the hospital where the highest rates of infection, highest impact of infection, and antibiotic resistance are likely to be found. These areas include ICUs, cardiothoracic surgery units, and hematology/oncology units. However, with the current scrutiny on HAIs, hospital-wide surveillance (i.e., concurrent surveillance throughout the hospital) is becoming more prevalent and has been mandated in some states. As more hospitals implement electronic medical records, hospital-wide surveillance has become less daunting from a resource perspective. For example, collection of device days (denominator data), which previously required a daily review of patients, often by an infection preventionist, can now be accomplished via extraction of data entered into the electronic record by the bedside nurse as part of the daily patient nursing assessment. It is important for hospitals to consider implementing surveillance outside of the ICU setting because the proportion of patients with invasive devices is increasing, and in many hospitals interventions to reduce infection have primarily been targeted to ICU patients. Although the rates of infection may be lower in the non-ICU setting, given that ICU beds typically make up a minority of beds in most hospitals, the burden of infections in the non-ICU setting may actually be higher. Hospitals with sophisticated information systems may be able to streamline surveillance through the development of computer-based algorithms that identify patients at highest risk for an HAI. Surveillance for some infections (e.g., bloodstream infections or infections with antimicrobial-resistant organisms) is primarily microbiology based; therefore, hospital-wide surveillance for targeted infections can be implemented relatively easily. The highest quality surveillance methodology for HAIs was developed by the CDC and is unit based, infection site specific, and risk adjusted (i.e., expressed in terms of device-specific denominators).13 Because the National Healthcare Safety Network (NHSN) methodology is the most widely accepted, hospitals that use it are able to compare their institutional rates to those of a large group of hospitals across the country. The NHSN has rapidly expanded from a network of slightly more than 200 hospitals in 200614 to nearly 3500 in 2011,10 primarily owing to mandatory reporting requirements by the Center for Medicare and Medicaid Services (CMS). Unit-based surveillance trends should periodically be reported back to the health care workers in the unit. Although HAI rates (e.g., bloodstream infections per 1000 catheter-days) are useful for interhospital comparisons and the analysis of institutional long-term trends, feedback to frontline providers is more meaningful when expressed as a raw number of infections (e.g., four central line–associated bloodstream infections in the past 3 months). Infectious diseases of public health importance should be reported to public health agencies, whose requirements vary by state. Increasingly, states are mandating surveillance for HAIs with public reporting and the CMS now also mandates reporting of some HAIs. The purpose of isolation is to prevent the transmission of microorganisms from infected or colonized patients to other patients, hospital visitors, and health care workers, who may subsequently transmit them to other patients or become infected or colonized themselves. Although isolation guidelines are based on current understanding of the mechanisms of the transmission of organisms, few well-controlled studies have been performed to demonstrate their efficacy. Because HAIs are relatively uncommon events, any study designed to demonstrate efficacy requires sample sizes that are often prohibitively large. Thus, studies evaluating the efficacy of infection prevention interventions often lack the power to allow one to conclude confidently that there has been a lack of effect (i.e., such studies have a high probability of type II error). Because patient isolation is expensive, time consuming, and uncomfortable for patients, impedes care, and generates large volumes of trash because of the use of disposable products, it should be implemented only when necessary. Conversely, failure to isolate a patient with a transmissible disease may lead to morbidity and mortality and may ultimately be expensive when one considers the direct costs of an investigation of an outbreak and excess length of stay and the indirect costs of lost productivity. The practice of isolating patients has moved from the requirement for separate infectious disease hospitals to separate wards for these patients and, ultimately, to providing precautions in the general hospital environment. In 2006, the American Institute of Architects, in its Guidelines for Design and Construction of Health Care Facilities, made single-patient rooms the standard.14 Hospitals that have single-patient rooms exclusively are able to isolate patients with transmissible diseases without disrupting patient flow.15 However, existing facilities often still have a significant proportion of double-patient rooms. In 2007, the CDC and the Healthcare Infection Control Practices Advisory Committee issued a revision of the recommended guidelines for isolation.16 These guidelines outlined a two-tiered approach: standard precautions, which apply to all patients, and transmission-based precautions, which apply to patients with documented or suspected infection or colonization with certain microorganisms. These guidelines are summarized in Table 300-2. Standard precautions are based on the assumption that any patient may potentially be colonized or infected with organisms that are transmissible. Therefore, standard precautions apply to all patients, in all settings, at all times. The essential elements of standard precautions are hand hygiene, personal protective equipment (gowns, gloves, masks, and eye protection), and safe needle practices. Because most HAIs are transmitted by contact, primarily via the hands of health care workers,17 hand hygiene remains the single most important means to prevent transmission of nosocomial pathogens. Compliance by health care workers remains suboptimal although improving through numerous efforts, including The Joint Commission’s mandate to measure hand hygiene via the National Patient Safety Goals program. The microorganisms on hands can be divided into transient flora and resident flora.18 The resident flora include organisms of low virulence (e.g., coagulase-negative staphylococci, Micrococcus, Corynebacterium) that are rarely transmitted to patients except when introduced by invasive procedures.19 They are not easily removed through hand washing. The transient flora, however, are important causes of HAIs. These organisms are acquired primarily by contact, are loosely attached to the skin, and are easily washed off. Thus, the purpose of hand hygiene in the hospital is to remove the transient flora recently acquired by contact with patients or environmental surfaces.18 In addition, HAIs have been attributed to bacterial contamination of artificial fingernails; therefore, health care workers should not wear them. Alcohol-based hand rubs have become the recommended agents for hand hygiene in the health care setting.19 In situations in which the hands are visibly soiled, washing with soap (antimicrobial or nonantimicrobial) and water is recommended. Soap and water is also preferred when caring for patients with Clostridium difficile infection (owing to the poor sporicidal activity of alcohols)20 or norovirus infection.21 Hand hygiene should be performed before and after contact with patients, before any aseptic task, after contact with inanimate objects in the patient’s surrounding environment, and immediately after removing gloves.22 Wall-mounted dispensers with alcohol-based, waterless hand rubs should be installed in all hospital and outpatient rooms. In areas where this is not feasible, individual health care workers should carry small containers of waterless agents. Technologic interventions to improve hand hygiene compliance include electronic dispensing counters, radiofrequency identification, alcohol vapor detection sensors, and videosurveillance.23 Gloves should be worn by health care workers to prevent contamination of the hands with microorganisms, to prevent exposure of the health care worker to bloodborne pathogens, and to reduce the risk for transmission of microorganisms from the hands of the health care worker to the patient. Standard precautions stipulate that gloves should be worn to touch any of the following: blood, all body fluids, secretions, and excretions, except sweat, regardless of whether they are visibly bloody, nonintact skin, and mucous membranes.20 Gloves should be changed during the care of a patient when moving from a contaminated body site (e.g., wound or perineal care) to a clean body site. However, gloves do not replace the need for hand hygiene. Contamination of the hands can occur with organisms on the surface of the gloves when they are removed, and some gloves have small perforations that may allow organisms to contaminate the hands. Thus, gloves should be viewed as an adjunctive protective barrier but not as a substitute for hand hygiene, which should be performed immediately after gloves are removed. For procedures that are likely to generate splashes or sprays of body fluid, a mask with eye protection or a face shield to protect the mucosa of the eyes, nose, and mouth, as well as a gown, should be worn. Disposable gowns should be made of an impervious material to prevent penetration and subsequent contamination of the skin or clothing. Standard precautions also stipulate that health care workers performing procedures involving lumbar puncture wear masks to prevent contamination of the spinal needle or the procedure site with the oral flora of the operator, which may occur when the operator is talking. Standard precautions also address respiratory hygiene, which includes instructing patients to cover their nose and mouth with a tissue when coughing or sneezing, performing hand hygiene after contact with respiratory secretions, placing a surgical mask on the coughing patient in common areas, and spatially separating patients with respiratory tract infections from other patients when feasible.20 Needles and syringes should be used only once and, when possible, single-dose medication vials should be used.24 Single-use vials of medication should not be used for more than one patient, and when possible multiuse vials should be assigned to a single patient. Intravenous solution bags should not be used as a common source of supply for more than one patient. Needles should not be recapped, bent, or broken but should be disposed of in puncture-resistant containers. Because of the potential for transmission for bloodborne pathogens, fingerstick devices for glucose monitoring should never be used for more than one person.25 If blood glucose meters must be shared, they should be cleaned and disinfected after every use. Insulin pens and other medication cartridges and syringes should never be used for more than one person. For the past several years, the National Health Service in the United Kingdom has mandated a “bare below the elbows” approach to patient care.26 To ensure optimal hand hygiene, this mandate requires that all health care workers wear either short-sleeved garments or long sleeves that are rolled up. In addition, wrist watches, bracelets, and rings with stones are not allowed. Neckties, if worn, must be kept tucked in. Transmission-based precautions apply to selected patients based on a suspected or confirmed clinical syndrome, a specific diagnosis, or colonization or infection with epidemiologically important organisms. Transmission-based precautions are always implemented in conjunction with standard precautions. Three types of transmission-based precautions have been developed for the major modes of transmission of infectious agents in the health care setting—airborne, droplet, and contact.20 A few diseases (e.g., varicella, severe acute respiratory syndrome) require more than one isolation category. Essential elements of each category are outlined in Table 300-2, and indications for implementation are delineated in Table 300-3. TABLE 300-3 Indications for Transmission-Based Precautions Acute diarrhea with likely infectious cause Respiratory tract infection in infants and young children* History of infection or colonization with MDRO† SSTI or UTI with recent stay in a facility where MDROs† are prevalent Abscess or draining wound that cannot be covered Cough, fever, any pulmonary infiltrate, and recent travel to regions with outbreaks of SARS or avian influenza*
Infection Prevention in the Health Care Setting
Historical Background
Role of Infection Control

Surveillance
Reporting
Isolation
Standard Precautions
Hand Hygiene
Gloves
Personal Protective Equipment
Injection Safety
Bare Below the Elbows
Transmission-Based Precautions
CONTACT PRECAUTIONS
DROPLET PRECAUTIONS
AIRBORNE PRECAUTIONS
Syndromes (Before Pathogen Identification)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

Infection Prevention in the Health Care Setting
300