INFECTION RATES
Because of their documented positive impact on HAI prevention and patient outcomes, surveillance and control programs aimed at HAIs have become priorities for healthcare providers across the United States. At its inception, a key objective of the managed, integrated care model was to improve patient outcomes through enhancement of the quality of medical care provided by hospitals through reduction of HAI occurrence while simultaneously controlling costs. To achieve this objective, the key components of the managed care business model included substantial downsizing of general hospitals, and monitoring the quality of care and the occurrence, effects, and outcomes of HAIs through the estimation of infection rates using approaches that are strikingly similar to the principles and systemic application espoused by Deming for the continuous quality improvement process in manufacturing (
5). These principles include the classification of manufacturing errors as either “special” or “usual” causes. For both manufacturing and healthcare services, the emphasis is on changes at the system rather than the individual level (
6).
The estimation of HAI rates in the United States began with surveillance studies of the prevalence and incidence of HAIs in individual hospitals (
7,
8,
9). The first systematic effort to estimate the magnitude of the problem on a wider scale was made by CDC in a collaborative study of eight community hospitals known as the Comprehensive Hospital Infections Project (CHIP) (
7). Performed in the late 1960s and early 1970s, this contract-supported study involved very intensive surveillance efforts to detect both HAIs and community-acquired infections. At that time, data from these surveillance efforts suggested that ˜5% of patients in community hospitals acquired ≥1 HAI, an estimate that was subsequently widely held to be the national HAI rate.
In 1970, CDC established the National Nosocomial Infections Surveillance (NNIS) system, for many years the only source of national data on the epidemiology of HAIs in the United States, the pathogens that cause these HAIs, and their respective antimicrobial susceptibility profiles (
10). The participating hospitals collected and reported to CDC their HAI data on patients using standardized protocols, called surveillance components: the adult and pediatric ICU, high-risk nursery, and surgical patient components (
10). In 2004, the NNIS system was combined with two other national healthcare surveillance systems—the National Surveillance System for Healthcare Workers and the Dialysis Surveillance Network—into a single Internet-based system—the National Healthcare Safety Network (NHSN) (
11).
The NHSN network comprises four surveillance components, each associated with HAI control and prevention—patient safety, healthcare personnel safety, biovigilance, and electronic surveillance (
12). In June 2007, NHSN released its first report on device-associated infections (
13). The data collected and reported by facilities participating in NHSN include risk-adjusted HAI data, adherence to clinical practices and procedures known to prevent HAIs, and incidence and prevalence of multidrug-resistant healthcare-associated infection pathogens within the respective facilities. The identities of all NHSN facilities are held confidential in accordance with Sections 304, 306, and 308(d) of the Public Health Service Act. This assurance of hospital confidentiality enhances the likelihood that active surveillance and accurate reporting of HAIs occur, because “name and blame” are removed from the surveillance system.
NHSN hospitals collect and report data on all sites of HAI in ICU patients (
13). In addition, ICU-specific denominator data are collected. Thus, site-specific and ICU-specific infection rates may be calculated and risk-adjusted using as the denominator the number of patients at risk, patient-days, or days of device use (e.g., days of indwelling intravascular catheters, urinary catheters, or mechanical ventilation). Because NHSN allows for a more uniform collection and analysis of data, several states, including California, Colorado, Illinois, Missouri, New York, Oklahoma, Pennsylvania, South Carolina, Tennessee, Vermont, Virginia, and West Virginia, require their facilities to report directly to the network. Currently, only 21 states require public reporting of hospital data on surgical site infections (SSIs), and even when disclosure is mandated, the information often is not easily accessible to patients. Aggregating institutions, other than CDC, that collect and report HAI rates include the following: Pennsylvania Cost Care Containment Council (PHC); the South Carolina Hospital Association; North Carolina Hospital Association; and the Duke Infection Control Outreach Network (DICON)—a collaboration between Duke University School of Medicine and a number of community hospitals.
During 1974 to 1983, CDC carried out the seminal Study on the Efficacy of Nosocomial Infection Control, more commonly known as the SENIC project (
4). One of the objectives of the SENIC project was to derive a more precise estimate of the nationwide HAI rate from a statistical sample of US hospitals (
14). The SENIC project was among the first to establish scientifically that HAI surveillance is an essential element of an effective infection control program. With 338 randomly selected general medical and surgical hospitals with ≥50 beds taking part, and examination of over one-third million patient medical records, the report from the SENIC project estimated that ≥2.1 million HAIs occurred among the 37.7 million admissions to the 6,449 acute care US hospitals during a 12-month period in 1975 through 1976 (
15). This gave rise to a nationwide overall infection rate of 5.7 HAIs per 100 admissions (the infection ratio): that is, ˜4.5% of hospitalized patients experienced ˜1 HAI (the infection percentage). Other key findings of the SENIC project included the following (
4):
Hospitals with the lowest HAI rates had both strong surveillance and prevention and control programs.
One-third of HAIs involving the four major anatomic sites (urinary tract, surgical wounds, respiratory tract, and bloodstream) that would otherwise occur in the absence of infection surveillance and control endeavors could be prevented by well-organized infection control programs.
The critical components of an effective HAI preventive program included a balance between surveillance and control efforts, at least one infection control practitioner for every 250 hospital beds, and a trained hospital epidemiologist.
HAI rates increased by an average of 3% annually in facilities that had not established infection surveillance and control programs.
Different categories of HAIs required specific control programs, whose effectiveness were not necessarily transferable when applied arbitrarily for control of any class of HAI.
Precise determination of the specific methods and schedules used in performing surveillance was not feasible largely because most of the participating hospitals were performing surveillance for infections at all anatomic sites across all hospital areas. These data suggested that hospital-wide HAI surveillance data had significant limitations that rendered them invalid for benchmarking.
Subsequently, scientific evidence from various other published studies has shown that surveillance activities do indeed reduce HAI rates. For example, the collection, calculation, and dissemination of surgeon-specific SSI rates to surgeons were shown to lower SSI rates in several published studies (
16,
17,
18,
19,
20). Currently, regulatory and accreditation agencies, such as The Joint Commission (TJC; formerly the Joint Commission on Accreditation of Healthcare Organizations [JCAHO]), and the Centers for Medicare and Medicaid Services (CMS) use HAI surveillance data to evaluate the quality of care provided by healthcare services. HAI surveillance activities enable healthcare facilities to analyze objectively and follow the trends of their own endemic HAI rates over defined time periods, and are now an integral component of systemic preventive efforts in healthcare facilities, including the acute care hospital inpatient setting, outpatient clinics, freestanding medical and surgical centers, long-term care facilities, and the home setting.
The CDC investigators recognized early on that the overall HAI rates, such as those cited, were crude rates (i.e., imprecise, meaningless, and not valid unless they were risk-adjusted). A crude overall HAI rate is the total number of HAIs at all sites (e.g., urinary tract infections [UTIs], pneumonias, SSIs, bloodstream infections [BSIs], and others) divided by a measure of the population at risk (e.g., the number of admissions, discharges, patient-days, or device-days). Using a crude HAI rate to characterize a hospital’s HAI problem has been seriously questioned or rejected (
21,
22). Many investigators and organizations, including the Task Force on Infection Control for the JCAHO, have rejected this rate as a valid indicator of quality of care (
23). The reasons were stated by Dr. Robert Haley himself, the task force chair and a principal investigator in the SENIC project: “A hospital’s crude overall nosocomial infection rate was considered to be too time consuming to collect because of the need to do continuous, comprehensive surveillance, unlikely to be accurate, and thus misleading to interpret, and unusable for interhospital comparison because of the lack of a suitable risk index of infection of all types” (
24). Before HAI rates are used for interhospital or intrahospital comparison or as indicators of quality of care, they require risk adjustment. As presently derived, a crude overall HAI rate of a hospital provides no means of adjustment for inpatients’ intrinsic or extrinsic risks and is meaningless. Thus, CDC has categorically stated that such a rate should not be used for interhospital comparison (
25).
For surveillance data to be used effectively, infection rates need to be calculated. An infection rate is an expression of the probability of the occurrence of an infection during a certain time interval. The numerator of an infection rate is always the number of infections of a particular type that have been acquired by a specific patient population over a defined time period. The group of patients chosen and the choice of the denominator used in calculating the infection rates are what separate comparative rates from those that are not. For HAI rates to be established as the basis for measuring quality of care, they must be meaningful for comparison either between healthcare facilities or within a single facility over time.
The concept of a comparable rate is one that controls for variations in the distribution of major risk factors (e.g., exposure to medical devices, or undergoing a surgical procedure) associated with the event so that the rate could either be monitored and analyzed meaningfully within the facility itself without reference to an outside standard or rate from another facility, or compared with an external standard or benchmark rate. Risk factors could be either intrinsic or extrinsic: the former includes congenital or hereditary disorders, underlying acquired conditions such as chronic cardiac or pulmonary disease, endocrine disorders, immunosuppression, age and gender, or high severity of illness scores. Extrinsic risk factors include various forms of medical and surgical therapies, procedures or interventions, exposure to antimicrobials or invasive medical devices (e.g., intravascular catheters, mechanical ventilators, urinary catheters, chest tubes, and ventriculostomy catheters), receipt of solid organs or allograft tissues, duration of hospitalization, or exposure to various healthcare personnel.
There are two types of rate comparisons—intrahospital and interhospital. The primary goals of intrahospital comparison are to identify areas within the hospital where HAIs are more likely to occur and may need attention and targeting of resources, and to measure the efficacy of interventional efforts. The quantification of baseline HAI rates enables hospitals to analyze and follow their HAI trends objectively. Intrahospital monitoring of HAIs has the advantage of better control of observer variation, especially for HAI case finding, culturing frequency and technique, and controlling for the case-mix of the patient population under study. Unfortunately, sample size comparison within a single facility can be a major problem, especially when monitoring HAI rates associated with surgical procedures. This limitation can be mitigated through participation in a surveillance system that aggregates data from multiple healthcare facilities, thereby enabling interhospital comparison of HAI rates.
Interhospital comparison (or comparison to an external standard or benchmark) entails comparing the rates with those of other hospitals participating in a multicenter surveillance system. Without external comparisons, hospital infection control departments may not know whether the endemic rates in their respective facilities are relatively high, or on what area to focus the limited financial and human resources of their infection control program. Moreover, since only about 10% of HAIs occur in recognized epidemics, the endemic infection rate in a facility may be steady and consistent so that variations that signal an outbreak may be absent (
26).
Like intrahospital comparison, one of the key objectives in comparing HAI rates of a hospital with those of other similar
facilities is to assess areas or infection control issues (or HAI rates) that might need attention. However, the approach is different. For any healthcare facility, external interhospital comparisons, while very appealing, may incur more limitations than intrahospital comparisons. For example, facilities participating in a multifacility surveillance system can be audited by the institution that aggregates the data to ensure that the data being reported is valid and meets the requirements of the surveillance system. Moreover, interhospital comparisons work on the basis and notion that a large number of hospitals are collecting data in the same, consistent manner, and are reporting these data in the same way to the aggregating institution. The differences in rates among hospitals are assumed by many to represent differences in healthcare worker (HCW) or institutional practices and procedures for preventing HAI. Whereas a relatively low HAI rate may be interpreted as an indication that the facility’s infection control program is effective at preventing HAI, the converse may also be true if HAI case finding is suboptimal, patient census for critically ill patients is relatively low, or there is selection bias in the reporting of rates that make the facility look better, especially if the surveillance system is passive. An HAI rate found to be relatively high compared with that of other hospitals may suggest a potential problem in the facility of concern; it does not, however, establish by itself that the problem is poor infection control since it may be a reflection of overzealous or inaccurate case finding, inaccurate aggregation of denominator data, or it may be merely a reflection of high patient census for a very sick inpatient population requiring critical care management in the ICU—that is, larger numbers of patients requiring invasive medical devices, mechanical ventilation, and prescribed antimicrobials.
Surveillance data generated from epidemiologic studies may be used to determine the need for clinical or public health action; assess and evaluate the effectiveness of prevention, intervention, or control programs, diagnostic algorithms, and prescribing policies; or set priorities for rational and appropriate use of limited microbiology resources, planning, and research. An understanding of epidemiology is important for quantifying and interpreting microbiology and pharmaceutical data, and for application of these data to clinical practice, quality assurance, hypothesis generation during investigation of outbreaks and other adverse events, rational prescribing policies, and public health.
HAI comparisons should be used only as an initial guide for setting priorities for further investigation. To be successful, multicenter HAI surveillance and monitoring systems must satisfy three requirements (
6): (a) the purpose must be clear; (b) the system must use standardized HAI definitions, data fields, and protocols; and (c) an aggregating institution must be identified to standardize definitions and protocols, receive the data, assess them for quality, standardize the risk adjustment of benchmarks, and interpret and disseminate the data to those who need to know (
6,
27,
28). CDC has remained the sentinel aggregating institution for active HAI surveillance in the United States since the 1960s.
RATES BY SITE OF HAI
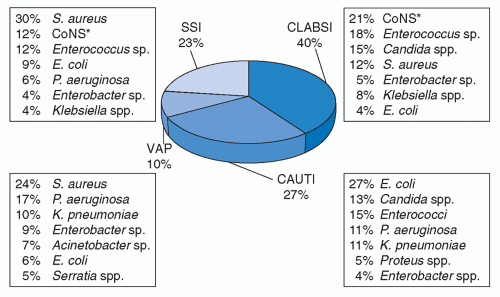
HAIs involve diverse anatomic sites. However, the relative frequencies of these infections will vary by site and by pathogen. The most common HAIs reported to NHSN are central line-associated bloodstream infections (CLA-BSI), 40%; catheter-associated urinary tract infections (CA-UTI), 27%; SSIs, 23%; and ventilator-associated pneumonia (VAP), 10% (
Figure 30.1) (
35). However, the overall HAI rates and relative frequencies of HAIs involving different sites tend to vary by type of ICU (
35). For example, the rates of CLA-BSI, CA-UTI, and VAP in medical-surgical ICUs are 21%, 18.5%, and 26%, respectively (
35). The corresponding rates for NHSN medical ICUs are 9%, 8%, and 10%, respectively; for NHSN surgical ICUs: 5%, 7%, and 15%, respectively; and for NHSN pediatric ICUs: 5%, 2%, and 4%, respectively (
35). Moreover, the distribution of infection sites and pathogens in pediatric ICU patients differ with age and from those reported from adult ICUs (
36).
Unlike ICU HAIs where one risk factor (medical devices) predominates, the risk of SSIs among patients who have undergone surgical procedures is related to a host of factors, including the operative procedure performed, the experience of the surgeon, the degree of microbiologic contamination of the operative field, duration of the operation, whether antimicrobial prophylaxis was administered at the most appropriate time before the incision, and the intrinsic risk of the patient (
15,
17,
18,
19,
37). An SSI risk index that effectively adjusts SSI rates for most operations has been developed by CDC (
38). This risk index uses a scoring system ranging from 0 to 3 and consists of scoring each operation by counting the number of risk factors present from among the following: (a) a patient with an American Society of Anesthesiologists (ASA) preoperative assessment score of 3, 4, or 5; (b) an operation classified as contaminated or dirty-infected; and (c) an operation lasting over
T hours, where
T is the approximate 75th percentile of the duration of surgery for the various operative procedures reported to the CDC database and depends upon the operative procedure being performed. The risk index is a better predictor of SSI risk than is the traditional wound classification system alone and performs well across a broad range of operative procedures. The risk index also predicts varying SSI risks within a wound class, suggesting, for example, that all clean operations do not carry the same risk of wound infection. The SSI rates should be stratified by risk categories before comparisons are made among institutions and surgeons or across time. The exceptions are spinal fusion, craniotomy, ventricular shunts, and Caesarean section operations in which SSI risk is not predicted by the risk index.
Healthcare-associated BSIs, especially CLA-BSIs, cause substantial morbidity and mortality. BSIs are either primary or secondary. The former are culture-documented BSIs in which no other site of infection was found to be seeding the bloodstream and usually ensue following direct infection. Of BSIs reported
to CDC, ˜64% are primary. Intravascular catheter use is the major cause of primary BSI. The microbiologic features of primary BSI have changed since the early 1980s. In 2004, CDC reported the highest mean rates (number per 1,000 central line-days) of CLA-BSIs in trauma ICUs (7.4), followed in descending order by burn ICUs (7.0), pediatric ICUs (6.6), and medical ICUs (5.0); the lowest BSI rates (2.7) were found in cardiothoracic units (
39). During 2009 to 2010, NHSN reported the highest CLA-BSI rates in medical-surgical ICUs (21%), followed in descending order in neonatal ICUs (10.5%), medical ICUs (9%), surgical ICUs (5.4%), pediatric ICUs (5.2%), cardiothoracic (4%), medical-cardiac (4%), trauma (3%), neurosurgery (1.5%), burn units (1.4%), and respiratory ICUs (0.1%) (
35).
On the basis of its microbiologic features, the pathogenesis of secondary BSIs (not included in
Figure 30.1) appears to be different from primary BSIs. The risk of secondary BSI is highest after lower respiratory infections (7.8%), SSIs (6.6%), or UTIs (4.4%). For SSIs, the probability of developing a secondary BSI varies with the primary site of infection—from 3.1% for incisional SSI to 9.5% for organ/space SSI (
40). Complications of infection by secondary BSI are most common on the cardiac surgery service (9.0%), followed by general surgery service (6.5%), the high-risk nursery (6.4%), the burn or trauma service (5.6%), and the urology service (4.9%). Secondary BSI was least likely on the otolaryngology service (2.6%), the orthopedic service (2.5%), and the gynecology service (2.3%). Secondary BSIs are more likely to occur in teaching hospitals. The organisms most commonly associated with secondary BSIs include
Staphylococcus aureus (20.9%),
Escherichia coli (11.3%),
Pseudomonas aeruginosa (9.6%), and
Enterococcus spp. (9.2%).
Among the various epidemiologic investigations that have characterized BSIs outside the acute inpatient setting, four have implicated the use of needleless devices as risk factors for acquisition of BSIs in home healthcare settings (
41,
42,
43,
44). The associated risk factors included receipt of total parenteral nutrition and use of multilumen catheters. DSN data from 109 participating hemodialysis centers reported that during 1999 through 2001, the vascular access infection rate per 100 patient-months was 3.2 overall and varied by type of vascular access: 0.6 for native arteriovenous fistulas, 1.4 for synthetic arteriovenous grafts, 8.4 for cuffed catheters, and 12.0 for noncuffed catheters (
45). NHSN data for 2006 indicate pooled mean rates of hospitalization among dialysis patients with arteriovenous fistulas, grafts, permanent and temporary central venous catheters to be 7.7, 9.2, 15.7, and 34.7 per 100 patient-months, respectively (
34). For BSI, the pooled mean rates were 0.5, 0.9, 4.2, and 27.1 per 100 patient-months in these groups, respectively. The microorganisms most frequently identified were common skin contaminants (
34).
HAI RATES BY PATHOGEN
NHSN data indicate that eight pathogen groups account for ˜82% of HAIs in US hospitals (
Table 30.1). The three most commonly reported pathogens are
S. aureus (15.6%),
Enterococcus spp. (13.9%), and
E. coli (11.5%). Compounding the issue is the fact that all eight pathogen groups demonstrate antimicrobial resistance to one or more commonly used antimicrobials (
35). Of the microorganisms that predominate among the four major infection sites—bloodstream, surgical wound, respiratory tract, and urinary tract—
S. aureus remains the most common cause of SSIs and hospital-acquired pneumonia, including VAPs; and
Enterococcus spp. are the third most common cause of SSIs (12%) and the second most common cause of BSIs (
35) (
Figure 30.1). Although coagulase-negative staphylococcus remains the most commonly reported cause of HAIs (
Figure 30.1), this rate of occurrence is probably inflated largely because coagulase-negative staphylococcus is a common skin commensal that also is a common blood culture contaminant. In a relatively recent study of positive blood cultures, although coagulase-negative staphylococci were the most common isolate, only 10% were clinically significant (
46). Not infrequently, a single blood culture that yields growth of coagulase-negative staphylococcus is deemed clinically significant when in fact it is not. Weinstein and colleagues have shown that when only a single blood culture set has been drawn and yields growth of
Staphylococcus epidermidis, the culture result almost always is (97.1%) likely to represent contamination (
47). Tokars et al. have shown that for blood cultures positive for coagulase-negative staphylococcus, the positive predictive value for clinical significance was 55% for one positive culture result of one culture performed, 20% for one positive result of two performed, and only 5% for one positive result of three performed (
48). In addition, he showed that for two positive culture results of two cultures performed, the positive predictive value is 98% if both samples were obtained through the vein (
48). That said, coagulase-negative staphylococcal BSIs remain one of the best markers of intravascular device-related infections in ICUs. Further studies and improvements in surveillance definitions and laboratory techniques are needed to further clarify the roles of coagulase-negative staphylococci, anaerobic bacteria, and viruses, whose true roles as causes of HAI have not yet been fully characterized.
Other data from the Surveillance and Control of Pathogens of Epidemiological Importance (SCOPE) study, a multicenter surveillance system for BSIs in the United States, have established that gram-positive organisms are associated with 65% of healthcare-associated BSIs in the United States while gram-negative organisms and fungi cause 25% and 9.5%, respectively (
49). The frequencies of bloodstream pathogens in the SCOPE study were as follows: coagulase-negative staphylococcus (31%),
S. aureus (20%),
Enterococcus spp. (9%), and
Candida spp. (9%)—consistent with or similar to CDC NNIS/NHSN data.
The increasing role of gram-negative pathogens as important causes of HAI was highlighted in a 2004 editorial (
50). For example, gram-negative BSIs predominate in patients with malignancies, burn patients with catheters, and patients with needleless intravascular devices (
42). Although
S. aureus remains the most common (24%) cause of VAP in hospitals that report data to CDC, the next six most common causes of VAP are gram-negative microorganisms:
P. aeruginosa (17%),
Klebsiella pneumoniae (10%),
Enterobacter spp. (9%),
Acinetobacter spp. (7%),
E. coli (6%), and
Serratia spp. (5%) (
Figure 30.1). In another analysis of CDC data, Gaynes et al. found that during 1986 through 2003, although gram-negative microorganisms were still commonly associated with HAIs in ICUs, especially UTIs (71%) and pneumonia (65%), the percentage of infections associated with gram-negative bacilli in the bloodstream and surgical wounds decreased from 33.2% in 1986 to 23.8% in 2003 and from 56.5% in 1986 to 33.8% in 2003, respectively. They also noted that although the percentages of pneumonia episodes and UTIs associated with gram-negative bacilli remained constant during the study period, the proportion of
Acinetobacter spp. associated with ICU pneumonias
increased from 4% in 1986 to 7.0% in 2003 (
51). More recent NHSN data show the following changes in antimicrobial resistance among pathogens that cause VAP during 2007 through 2010:
S. aureus resistance to oxacillins (51.9% to 48.4%);
Enterococcus faecalis to vancomycin (6.4% to 9.8%);
Klebsiella spp. to extended-spectrum cephalosporins (21.5% to 23.8%);
E. coli to extended-spectrum cephalosporins (14.2% to 16.3%); multidrug-resistant
P. aeruginosa (16.6% to 17.7%);
Acinetobacter spp. to carbapenems (56.7% to 61.2%).
RISK FACTORS AND DETERMINANTS ASSOCIATED WITH HAIS
The strongest determinants of HAI risk are the characteristics and exposures of patients that predispose them to infection and the complex interactions of the agent (microorganism causing infection), host (susceptible patient), and environment (hospital ICU, outpatients, hemodialysis center, surgery or medical centers, or home). The agent, host, and environment make up a triad that is a useful model for the characterization of infectious disease epidemiology in healthcare and other settings (
52). In this model, the environment is the backdrop against which a microorganism interacts with a susceptible patient to cause infection.
The probability of infection depends on both the microorganism and the host: for the former, the intrinsic characteristics and properties (infectivity, pathogenicity, and virulence) of the microorganism; for the latter, it is increased if the host is immunocompromised, nonimmunized, or unvaccinated. Other agent factors that are important for the development of disease include the infecting dose, its ability or propensity to produce toxins, its immunogenicity and ability to resist or overcome the human immune defense system, and its ability to replicate only in certain type of cells, tissues, or patients. Other intrinsic and genetically determined properties of a microorganism may be important for it to survive in the host or environment. In the inpatient setting, these include the organism’s response to the effects of heat, drying, disinfection or sterilization, or antimicrobials; its ability to compete with other microorganisms within the host or the environment; and its ability to independently multiply in the environment (
52).
For transmission and infection to occur, the microorganism must remain viable in a reservoir or the environment until direct or indirect transfer to a susceptible host and contact
with the host has been sufficiently long enough to cause infection and disease. The entire transmission process constitutes a chain of infection. If this chain of infection remains unbroken, the size of the reservoir may increase in the continuing chain of transmission. Examples of reservoirs that allow the agent to survive or multiply include HCW carriage of
S. aureus in the anterior nares,
P. aeruginosa under false fingernails,
Serratia marcescens in soap preparations or damp areas around sinks,
Legionella spp. in central humidifiers of air-conditioning systems that disseminate the organism through the air in droplet nuclei, drug preparations or dialysis fluids that are intrinsically contaminated at the manufacturer, multidose vials that become contaminated during access with a needle and syringe (this becomes a major problem if numerous patients receive fluid from a single contaminated multidose vial), or sterile infusates that become extrinsically contaminated within the acute care setting (e.g., wards or hospital pharmacy) (
31,
53,
54,
55,
56,
57,
58).
Indirect contact transmission is the most common mechanism of transfer of the microorganisms that cause HAIs, and commonly occurs via the hands of HCWs. Other examples of indirect contact transmission include contaminated inanimate objects (fomites), work surfaces, and biological fluids (e.g., respiratory, salivary, gastrointestinal, or genital secretions, blood, urine, or stool). Medical devices contaminated with blood-borne pathogens (e.g., hepatitis B and C viruses, cytomegalovirus, or HIV) are sources of infection for both patients and HCWs in hospitals, outpatients, long-term care facilities, or the home. In pediatric populations, fecal-oral spread is an important means of indirect contact transmission of a variety of bacterial, viral, and parasitic infectious diseases. The mechanisms are commonly stool-hand-mouth or stool-object-mouth. Thus, in the United States, rotavirus and astrovirus are commonly implicated as the cause of hospital-acquired infectious gastroenteritis in pediatric inpatient populations (
59,
60). The airborne transfer of droplet nuclei remains the principal route of transmission of
Mycobacterium tuberculosis, varicella (chicken pox), measles, and
Legionella spp.
Patient factors (e.g., age, state of debilitation, immune or nutritional status, device usage, invasive procedures, or antimicrobial therapy use) play important roles in determining whether or not a patient will acquire an HAI. Special units for intensive medical or surgical care and for extensive burns, trauma, transplantation, and cancer chemotherapy frequently house patients who are susceptible to infection and disease caused by endemic organisms. In these patients, reduced inocula of pathogens may cause infection and disease, and nonpathogenic agents (e.g., coagulase-negative staphylococcus) may cause serious disease or death. Frequent opportunistic infections in these patients require repeated, broad, and extended therapy with multiple antimicrobials, leading to increasingly resistant resident microbial populations. Commensal microorganisms can become opportunistic pathogens under appropriate conditions. Patients with immunosuppression (e.g., patients with hematology conditions or HIV infection or who have had solid organ or bone marrow transplants or are receiving antineoplastic drugs) are at high risk of opportunistic bacterial, fungal, or protozoal infections.
Whether an infecting agent produces clinical or subclinical infections also depends on the agent and certain host factors (e.g., age and immune status.). For example,
P. aeruginosa, a ubiquitous pathogen that thrives in aquatic environments, soil, and vegetation, seldom causes disease in healthy populations. However, in debilitated populations, such as those with burns, malignancies, leukemia, critical care patients with multiple
in situ invasive medical devices, or children with cystic fibrosis, this pathogen remains a significant cause of VAP and CLA-BSI (
36,
61,
62).
Over the past three decades, much epidemiologic and clinical research have been carried out, either through formal studies or outbreak investigations, to characterize the risk factors associated with the occurrence of HAIs in various US healthcare settings. However, it has not always been clear whether risk factors identified in these studies or investigations were the true underlying cause of the infection or were merely associated with the HAI event. Undoubtedly, some risk factors are the direct cause of infection while others are only coincidentally associated with the event because they follow infection or are merely surrogate markers for the intrinsic risk factors associated with the patient or the microorganism. Complicating matters further is the fact that ≥2 independent risk factors often occur simultaneously in the same patient, sometimes exerting additive or even synergistic effects. Such risk factors are considered to be strongly intercorrelated.
NNIS and NHSN data indicate that HAI occurrence in ICUs continue to remain unacceptably high (
31,
13,
35,
36,
61,
62,
63,
64,
65). The reasons for this continuing problem are varied and complex and include the following: (a) increased ICU patient census due to greater need for intensive care, (b) a greater number of susceptible patients (e.g., the very young or elderly, and those with severe underlying disease, burns, malnourishment, or immunosuppression) being admitted to ICUs, (c) increased use of invasive medical devices in ICUs, (d) lapses in infection control, (e) crowding or decreased nurse-to-patient ratio in the ICU, or (f) increased presence of HAI pathogens in the environment (
66,
67,
68,
69).
Environmental factors, the third component of the triad, facilitate the transmission and acquisition of HAI through three principal modes of interaction with the agent and host that determine infection or disease (i.e., agent-host, agent-environment, and host-environment interactions). The relative contribution of each of these interactions to the acquisition and pathogenesis of infection or disease is rendered complex because of the wide variety of infectious agents, hosts, environmental factors, and variability of parameters that make up each of these components. For example, CDC data suggest that the ICU is the area of highest risk for the transmission of HAIs (
31). Moreover, methicillin-resistant
S. aureus (MRSA), vancomycin-resistant enterococcus (VRE), and
P. aeruginosa already are endemic in the ICUs of many hospitals that report HAI data to CDC (
31,
70). A complex interaction of concomitant factors, such as a pathogenic microorganism that is already endemic in the ICU environment, an inpatient population of susceptible patients, inadequate adherence to hand hygiene or infection control practices among healthcare personnel, fluctuating staffing levels, unexpected increases in patient census relative to staffing levels in the ICU, or an unexpected increase in the number of severely ill patients with multiple invasive devices might also contribute to HAI acquisition (
66,
67). Adding to the complexity of the entire process is the potential transmission of the agent from host to HCW, HCW to HCW, or host to environment. Thus, acceptable measures for HAI prevention and control measures dictate that the hospital epidemiologist or IPs identify and analyze the interrelationships among all components of the triad of agent, host, and environment.
It is well known that the social environment is extremely important in determining human behavior that affects the direct transmission of microorganisms (e.g., artificial nails worn by HCWs in ICUs) (
53). Equally relevant is the impact of other social factors (e.g., distribution of and access to medical resources), use of preventive services or enforcement of infection control practice recommendations, acceptance of advice or guidelines on the appropriate use of antimicrobials, or appreciation by relatives, patients, and healthcare personnel alike that patients who are aged, severely ill, born prematurely, or have congenital abnormalities, have numerous indwelling medical devices, or have had multiple invasive procedures or surgery will be especially susceptible to HAIs. Finally, there must be a willingness of all involved to appreciate the limitations of medical technology and antimicrobials when all other clinical evidence and experience suggest that the condition of the sick person is irretrievable.
To design strategies for preventing HAIs, it is important to differentiate among coincidental indicators of risk, independent causal factors, and synergistic interactions of causal factors. In a study of 169,526 patients who made up a representative sample of patients admitted to acute care US hospitals in 1975 and 1976, population estimates of HAI rates for each of the four major types of infection were calculated within each category of exposure to between 10 and 20 separate risk factors (
4,
19,
71). A striking finding was that all of the risk factors were associated with HAI at all four major anatomic sites. At first, this seems surprising because one would not expect a direct causal association between mechanical ventilation, for example, and acquiring a UTI. The explanation, of course, is that some of the associations indicate direct causal relationships; others indicate partial causal relationships, potentiated or diminished by other concurrent influences; and others (such as that between respirators and UTI) represent largely coincidental associations (most patients on respirators also have indwelling urinary catheters that predispose them to CA-UTIs).
The two factors that appeared to exert the strongest causal influences in all four sites of infection were indicators of the degree of the patient’s underlying illness: (a) in surgical patients, the duration of the patient’s operation, and (b) an index of the number and type of distinct diagnoses and surgical procedures recorded (intrinsic risk index). After these, several factors were strongly associated with infections at one or two sites but not with all four. Having a combined thoracoabdominal operation was strongly associated with pneumonia and SSI; undergoing a “dirty” (or contaminated) operation was associated with SSI; having an indwelling urinary catheter was an independent risk factor for acquiring a UTI; being on a respirator, with VAP or BSI; and having a previous HAI or receiving immunosuppressive therapy were both associated with BSI. Examples of risk factors that had weaker associations with all four sites were age, gender, previous community-acquired infection, and length of preoperative hospitalization.
Multivariate modeling has demonstrated that the risk of HAI is primarily determined by definable causal factors reflecting the patient’s underlying susceptibility to infection or the degree to which the microorganisms have access to vulnerable body sites. Modification of >1 of these factors can alter a patient’s risk. Multivariate statistical models based on measurable risk factors can be developed to predict accurately a patient’s risk of acquiring an HAI.
Colonization is the presence of a microorganism in or on a host with growth and multiplication but without any overt clinical expression or detected immune reaction in the host at the time the microorganism is isolated. In a colonized patient, an infectious agent may establish itself as part of a patient’s flora in multiple or specific anatomic sites or may cause low-grade chronic disease after an acute infection. Under suitable conditions, various patient populations colonized with
S. aureus are at an increased risk of developing infection and disease (
72,
73,
74). For example, nasal colonization with
S. aureus may be a risk factor for SSI in pediatric patients undergoing heart operations or for catheter-related infections in pediatric patients on chronic peritoneal dialysis (
75,
76). The HCW’s hands, colonized with gram-negative pathogens, such as
S. marcescens or
P. aeruginosa, may become potential sources of outbreaks in neonatal ICUs (
53,
54).