Keywords
bortezomib, chemotherapy, dexrazoxane, fertility preservation, in vivo animal models, mice, non-human primates, ovary, ovarian toxicity, ovoprotectant
5.1
Introduction
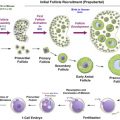
The success of cancer treatment creates a growing population of female cancer survivors who wrestle with long-term side effects of chemotherapy, including primary ovarian insufficiency (POI). This growing survivorship presents researchers and clinicians with the challenging mandate to develop therapeutics that improve ovarian function post-chemotherapy. Continuing research is therefore devoted to developing non-invasive, drug-based ovarian shields and ovarian-regenerative technology. An ideal ovoprotective drug would be easily administered in conjunction with traditional chemotherapy, and preserve both long-term endocrine function and fertility by preventing chemotherapy toxicity to the ovary. Developing ovoprotective drugs requires detailed understanding the mechanism(s) of chemotherapy ovarian insult in order to identify potential targets to achieve the ultimate balancing act: shield the healthy ovary from chemotherapy, but kill cancer. Key parameters include identifying the ovarian cell type(s) and follicles affected by each chemotherapy agent, and temporal resolution of primary insult and subsequent cell death. In vivo animal models are required to understand systemic effects of chemotherapy as they provide native cellular interactions, the dynamic intrafollicular communication and allow assessment of fertility and the health of subsequent generations.
5.2
Rodent Models of Chemotherapy-Induced Follicular Depletion and Ovoprotection
The most common in vivo model for ovotoxicity is the mouse. Rodents provide short lifespans with rapid progression from birth through reproductive maturity (weeks) and short intergenerational times (months), allowing comprehensive studies from acute insult through the birth of multiple generations. While not currently standard in ovotoxicity studies, transgenic mouse cancer models of leukaemia, breast cancer and sarcomas will be key in determining whether chemotherapy remains effective when administered with ovoprotective agents by providing simultaneous assessment of cancer remission rates and ovoprotection. Despite these advantages, large differences remain compared to human reproductive physiology; rodents are multi-ovulatory and typically do not experience natural menopause. Rodents are more sensitive to chemotherapy than humans, limiting cumulative chemotherapy doses and impeding recapitulation of multi-dose chemotherapy regimens. Proof-of-concept oncofertility rodent studies therefore require confirmation in non-human primates more closely related to humans.
5.2.1
Chemotherapy-Induced Follicular Depletion
Foundational chemotherapy studies measured ovotoxicity as follicle loss, leading to three models describing follicular depletion. In the first model, chemotherapy directly destroys primordial follicles, depleting the ovarian reserve. For example, cisplatin depletes primordial and primary follicles in mice treated at postnatal day (PND)5 (i.e., paediatric mice). Secondary follicle counts are unchanged at PND9, however, demonstrating relative resistance.
In the second model, chemotherapy induces apoptosis only in follicles containing actively dividing granulosa cells. Doxorubicin (DXR) induces widespread apoptosis in granulosa cells of growing follicles within 12 hours, but primordial follicles do not exhibit apoptosis until 48 hours post-treatment, and then only in small numbers consistent with the primordial follicle recruitment rate in mice.
The third, not mutually exclusive, “burnout” model postulates that cyclophosphamide first depletes growing follicles, which promotes primordial follicle recruitment, explaining the observed time-dependent follicular depletion followed by restoration of growing follicle numbers. This model predicts that consecutive chemotherapy treatments compound depletion as primordial follicles prematurely activated by the first round of chemotherapy become the growing follicle pool destroyed by the following chemotherapy dose. Future studies tagging chemotherapy-injured follicles to quantify growth and atresia in vivo may allow clear delineation of ovarian remodelling.
5.2.2
Cell-Type Specificity of Chemotherapy Toxicity
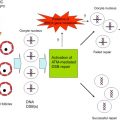
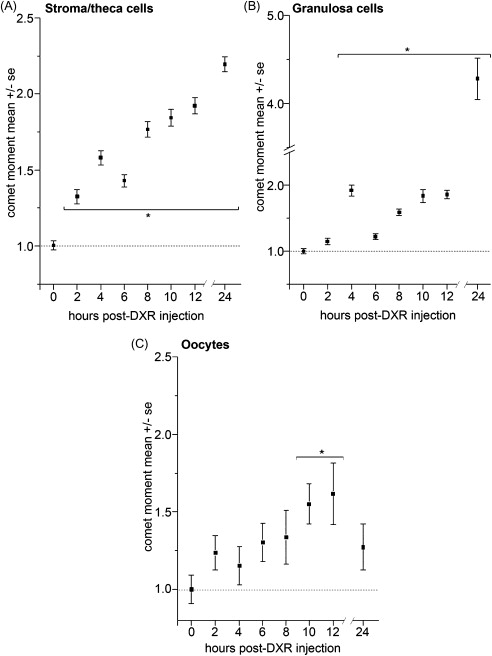
Early studies of chemotherapy toxicity placed significant focus on oocytes, including cisplatin destruction of primordial follicle oocytes. Distinct chemotherapy agents exhibit differential toxicity profiles, however, with respect to which cell and follicle types are the primary targets for insult and demise. Stromal and thecal cells in direct contact with the blood supply are the first cells to exhibit DXR-induced DNA damage, followed by subsequent damage to follicular cells ( Figure 5.1 ). Identifying the cells that receive the first insult for each chemotherapy agent is an important step toward designing protective approaches that prevent ovotoxicity.
5.2.3
Ovarian Toxicity Lessons from Ovoprotection Studies
Mechanistic details of how chemotherapy causes ovarian damage continue to expand through studies testing ovoprotective agents. Here we highlight key considerations for rodent models, including animal age at treatment, drug preparation methods and drug dose, alongside the need to develop robust chemoprotective agents that provide protection across a range of chemotherapy doses.
The importance of drug preparation was revealed by conflicting results in cisplatin-induced ovarian damage in studies by Gonfloni and Kerr. A thorough and mechanistic study by Gonfloni et al. demonstrating that imantinib shields the ovary from cisplatin damage showed an initial 40–50% loss of fertility and pups per litter following cisplatin. In replicating that study, however, Kerr et al. observed almost complete loss of both. Given identical calculated drug doses, mouse treatment and mating age, and that the Gonfloni group replicated their own results, differences in cisplatin formulations may account for higher level of toxicity and the resultant lack of imatinib protection observed by Kerr et al.
Comparing ovotoxicity across studies can be challenging when different chemotherapy doses are used. Cyclophosphamide at 50 mg/kg causes 60% primordial follicle loss in mice treated at 5–6 weeks of age. Higher cyclophosphamide doses (120 mg/kg) combined with busulfan (12 mg/kg) cause a striking >95% reduction in primordial follicle counts. While greater toxicity with higher dose is not surprising, these studies demonstrate challenges in cross-study comparisons and effective ovoprotective agents will need to shield across a range of chemotherapy doses.
Similar to cancer patients, age of mice at time of treatment alters the characteristics of chemotherapy ovotoxicity. The burnout model explains dynamics of initial secondary follicle depletion and restoration in PND5 mice treated with 150 mg/kg cyclophosphamide. In contrast, primordial follicles from mice treated at 4.5 weeks of age with 50 mg/kg cyclophosphamide were depleted prior to loss of growing follicles. While the chemotherapy dose was different, altered follicular dynamics are likely age-dependent. Similarly, 4-week-old (adolescent) mice pretreated with granulocyte colony stimulating factor as an ovoprotective agent exhibited >80% follicle loss following cyclophosphamide/busulfan, but breeding mice only showed a 30% reduction in litter size. These discrepancies may be due to the treatment of 8-week-old (reproductively mature) mice for fertility assessment. As the field moves forward, systematic assessment of age-related chemotoxicity and efficacy of ovoprotective agents will aid translation of fertility interventions to cancer patients.
5.2.4
Rodent Studies of Ovarian Chemotherapy Toxicity Compared to Data from Clinical Studies
Chemotherapy agents have been classified as differentially ovotoxic based on retrospective analysis of female cancer survivors who received multi-drug treatments. Alkylating agents like cyclophosphamide are considered the most detrimentally ovotoxic. Platinum drugs, including cisplatin, are classified as moderately ovotoxic, and anthracyclines like DXR, mildly ovotoxic. Rodent studies of cisplatin and cyclophosphamide confirm the human ovotoxicity, but mice receiving a single human equivalent dose of DXR exhibit a 50% decrease in ovarian mass and decline in fertility rates much greater than “mildly ovotoxic” would predict. It is unclear whether the disparities are due to species differences or the fact that infertility post-chemotherapy is underestimated, given that it requires self-reporting from patients, and that follow-up is usually for a relatively short period of time. In addition, clinical ovotoxicity has thus far only been scored according to infertility, not accounting for additional negative ramifications in females who do achieve pregnancy, including delivery complications and low birth weights. Sibling studies conclude that while alkylating agents (considered highly ovotoxic) do not affect birth weight, non-alkylating agents, particularly DXR, carry high risk for low birth-weight babies in subsequent pregnancies. These data highlight the need for a comprehensive classification of chemotherapy ovotoxicity.
Cancer patients receive chemotherapy cocktails, but most animal models have assessed ovotoxicity of each chemotherapy agent in isolation. Advancing toward the goal of cross-chemotherapy ovoprotection, tamoxifen shields the ovary from both cyclophosphamide ( in vivo ) and doxorubicin ( in vitro ). This study provides an important step toward animal models of combined chemotherapy regimens.
Another critical question in predicting POI following chemotherapy is determining how follicle counts predict POI and developing in vivo follicle assessment tools. Mice treated with cyclophosphamide exhibit a linear relationship between dose and primordial follicle depletion but, surprisingly, depleting 60% of primordial follicles does not change ovulation, fertilization, or pregnancy rates. Certainly follicle depletion reduces the fertile window, but in women, removing one ovary does not dramatically change the age of menopause. The processes allowing one ovary to compensate for the loss of the contralateral ovary are unknown, and may reveal novel ways to prolong fertility despite follicular depletion. Magnetic resonance imaging (MRI) has revealed DXR-induced ovarian remodelling in mice as a decrease in ovarian dimensions through 1-month post-DXR treatment. Future studies correlating follicle counts with ovarian changes measured by MRI may provide a non-invasive measure of ovarian remodelling and POI assessment post-chemotherapy in patients.
5.2.5
Transgenerational Toxicity of Chemotherapy
Current dogma suggests that women who become pregnant post-cancer are at no increased risk for offspring with congenital defects. Data from mice, however, demonstrate that while the first generation post-chemotherapy manifests no gross defects, chromosomal abnormalities appear in later generations. In a transplant model that distinguishes between effects on the ovary itself and general physiological decline of the dam, ovaries from DXR-treated mice were transplanted into naïve mice, which were subsequently mated. Starting at generation (G)4, DXR offspring exhibited increased neonatal death and physical abnormalities due to deletions on chromosome 10. Dams receiving DXR ovaries suffered frequent dystocia not observed in mice receiving control ovaries, and died due to delivery complications in G4–G6. Similarly, oocytes retrieved from cyclophosphamide-treated mice exhibited abnormalities that led to reduced fertilization and embryonic development, and increased aneuploidy. These data suggest transgenerational effects of chemotherapy may be underestimated in human cancer survivors, as society has not reached five generations post-chemotherapy (as these agents were first developed in the 1960s) and demonstrate that successful ovoprotective agents will need to prevent chemotherapy-induced genotoxicity, diminishing risk for propagating chemotherapy-induced genetic abnormalities.
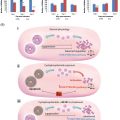
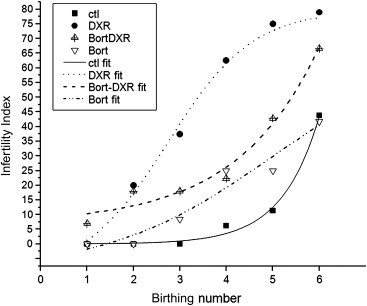
Mice provide the ideal model for initiating studies to determine whether mechanistic ovoprotective agents like dexrazoxane and bortezomib can prevent chemotherapy-induced genotoxicity and limit risk for future generations. Demonstrating the strengths of an in vivo model, including thorough analysis of acute insult to all ovarian cell types combined with long-term fertility assessment, a recent study by the authors showed that bortezomib shields granulosa, stromal/thecal cells and oocytes of adolescent mice (4 weeks of age) from DXR-induced DNA damage in a temporal fashion, preserving the growing follicle pool. While bortezomib did not alter initial DXR-induced loss, litter size recovered over time compared to DXR alone ( Figure 5.2 ). The temporal data provided a thorough assessment of fertility across the reproductive lifespan of the mice and revealed that initial fertility loss can be recovered. Future studies will determine whether mechanistic ovoprotective agents fulfil the potential of diminishing the transgenerational consequences of chemotherapy.
5.3
Primate Models of Chemotherapy-Induced Ovarian Toxicity and Ovoprotection
Rhesus macaques recapitulate the human reproductive system with the greatest fidelity and therefore represent a preferred animal model for validating ovoprotective agents. Macaque monkeys ovulate one oocyte approximately every 28 days, and menstruate ~14 days after ovulation. They reach puberty at 5–6 years of age, often achieve conception during their first ovarian cycle and have an average 176 day gestation period. However, reproductive studies require years to follow from treatment through breeding and subsequent generations. A complete cadre of assisted reproductive technologies (ARTs) have been implemented in rhesus monkeys, including ovarian stimulation, in vitro oocyte maturation, in vitro fertilization, intracytoplasmic sperm injection, embryo culture and embryo transfer. These techniques would easily allow acute examination of follicular and oocyte function, as well as embryonic effects, pre- and post-chemotherapy with an ovoprotective agent. Mechanisms of chemotherapy and ovoprotective agents should be studied in ovaries from animals of varying ages, which is critical given that ovarian reserve and response to anti-cancer therapies are age-dependent.
Marmoset monkeys may provide another model for ovarian toxicity, offering a shorter intergenerational time than macaques. Marmosets form stable families and ovulate 2–3 oocytes every 28 days. Adolescent female marmosets at 11–13 months are developmentally equivalent to adolescent human girls at puberty. Small in size, marmosets achieve sexual maturity earlier than macaques, with first conceptions occurring around 18 months and first births at 24 months of age. These short intergenerational intervals provide a translatable in vivo primate model for examining fertility and transgenerational endpoints following ovarian chemotoxicity and ovoprotection. ARTs in marmosets are not well developed, however. In vitro fertilization has been a particular challenge due to inadequate gonadotropin response, lack of ovarian ultrasound imaging during gonadotropin stimulation, and poor fertilization rates and embryo quality compared to rhesus macaques and women.
Non-human primates have been used in longitudinal chemotherapy toxicity studies lasting over 30 years. Despite similarities between the non-human primate and human ovary, challenges remain in using these models for radiotherapy and chemotherapy ovarian toxicity. Non-human primates have lower tolerability and survivability to chemotherapy than humans; chemotherapy doses routinely given to children can cause a myriad of complications in non-human primates. Standard human equivalent doses of DXR cause morbidity in macaques, such that relatively low doses at 1–2 mg/kg are routinely used, with a maximum of 5.83 mg/kg dose, which is <50% human equivalent. Similarly, rhesus monkeys treated with a single dose (4 to 12 mg/kg) of the alkylating chemotherapy, busulfan, manifested significant dose-dependent testicular insufficiency. Busulfan is used in humans at a total dose of 16 mg/kg (4 mg/kg daily for 4 days) for conditioning prior to bone marrow transplant. The 16 mg/kg total human dose is equivalent to around 50 mg/kg in the rhesus (based on Km=37 for a 60 mg adult with a body surface area of 1.2 m 2 and rhesus Km=12). Rhesus monkeys showed increased mortality due to severe bone marrow suppression at the lower 8 mg/kg dose.
Although non-human primates cannot tolerate precise human equivalent doses, developing ovoprotective agents requires defining the tolerable maximal dose that elicits ovarian toxicity mimicking the response in women who receive systemic chemotherapy. To maintain animal health, low doses of systemic chemotherapy must consistently be used in conjunction with vigorous prophylactic supportive care, e.g., apheresis. Administration via arterial vessels localized near the ovary or intraovarian delivery systems may circumvent detrimental systemic side effects of chemotherapeutic agents while maintaining ovarian toxicity and providing routes for simultaneous treatment with ovoprotectants. Although malignant lymphomas and leukaemias have been described in macaques and marmosets for many decades, the majority do not arise spontaneously, but are reported in animals with simian immunodeficiency virus-related immune suppression and associated with viral infections. Burkitt’s type and T-cell lymphomas can be virally induced (i.e., via lymphocryptovirus and simian T lymphotropic virus, respectively), but viral associated leukaemias are very rare. Thus, non-human primates are limited as naturally occurring cancer models, but haematological disease can be induced with oncogenic viruses. Despite these challenges, establishing non-human primate models to pre-clinically evaluate chemotherapy-induced ovarian toxicity in vivo is a priority if development and delivery of ovoprotective agents shown to be promising in rodents are to become a clinical reality.
One proof-of-concept study for ovarian protection based on previous mechanistic studies in rodents has been performed in female rhesus macaques, wherein intraovarian administration of the anti-apoptotic agent, sphingosine-1-phosphate (S1P) or its long-acting analogue, FTY720, preceding ovarian X-irradiation at a dose causing ovarian failure (15 Gy) sustained a cohort of preantral follicles. Ovarian cyclicity resumed in treated animals, and fertility was restored resulting in live offspring devoid of chromosomal abnormalities. The protective effect of S1P against ovarian failure caused by chemotherapeutic agents was corroborated by subsequent in vivo studies in rodents exposed to the alykylating agent dacarbazine, as well as human ovarian cortex treated with cyclophosphamide or DXR. The precise cellular targets (vascular, somatic, oocyte) and protective mechanism of S1P in the primate ovary are unknown. A disadvantage of S1P is that is must be delivered locally within the ovary and not systemically where it could potentially protect malignant cells from chemotherapy. Nonetheless, these studies demonstrate the utility of the non-human primate model for in vivo studies on prevention of ovarian damage from clinical cancer therapies by ovoprotectants that are potentially effective in women.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree