Keywords
animal models, apoptosis, chemotherapy, culture, follicle, in vitro , ovary, primary effects
6.1
Introduction
Investigating the precise effect of chemotherapy on female fertility is a key research aspect underpinning fertility-preservation work. Protecting the ovarian follicle pool will require detailed understanding of precisely how the different chemotherapy drugs damage the ovary: whether chemotherapy drugs directly kill oocytes or whether damage is first to surrounding somatic cells; if the drugs are equally toxic to all follicles or if specific stages of follicle development are particularly vulnerable to damage. The more information that is available, the more informed will be our development of clinical treatments to protect the ovarian reserve. So far, in vitro techniques have been used relatively little for research into effects of chemotherapy treatment and potential ovarian protection against damage, but work to date indicates that these are, in general, powerful tools for reproductive toxicology studies, and so also likely to be very useful here.
6.2
Why Use Culture Systems?
Tissue culture systems have been extensively used to study key mechanisms and processes in the ovary, including follicle recruitment, oocyte–somatic cell interactions and follicle–follicle interactions. There are a variety of culture systems available, short- and long-term, most covering specific stages of follicle development. The addition of chemotherapeutic drugs into such systems allows determination of the effect of these agents on the health of various components of the ovary. Depending on the aims of the study, the molecular mechanisms of cell and follicle death can be established, along with examination of the effect on individual ovarian cell types, specific follicle stages and elucidation of primary versus secondary effects. A major consideration when studying the ovary is that the follicle population within it is heterogeneous, with follicles in varying stages of development: in postpubertal females, this includes follicles from the primordial right through to the preovulatory stage. This complicates in vivo research into the effect of chemotherapy drugs, since it is difficult to ascertain the initial site of damage following chemotherapy exposure. In vitro models can span various, yet precise, stages of follicle development, allowing for analysis of specific cell and follicle types.
While in vitro models can maintain follicle–follicle interactions and the influence of stromal cells essential for physiological ovarian function, culture does, of course, involve isolating the ovary from its neuroendocrine inputs, which also impacts on follicle recruitment and growth. In vitro models are therefore useful for determining the direct effect of chemotherapeutic agents on the ovary, but do not allow examination of indirect ovarian effects, such as occurs following hypothalamic/pituitary damage during cranial irradiation.
When using in vitro techniques to examine the effects of chemotherapeutic agents, there are several limitations that must be considered. Premenopausal women receive complicated treatment regimens often involving combinations of several different drugs, generally given repeatedly over many weeks, which can be difficult to replicate. It is also hard to extrapolate the amount of drug that reaches the ovary solely from known serum concentrations, making it challenging to determine clinically relevant dosages to use in vitro . Chemotherapeutic agents can be metabolized in vivo , often in sites away from the ovary, such as the liver or the kidney, with the metabolites potentially having differing levels of ovotoxicity. An example of this is cyclophosphamide, which is metabolized by hepatic enzymes into a range of active metabolites including phosphoramide mustard and acrolein. Utilizing the exact combination of metabolites that the ovary could be exposed to is therefore difficult to simulate in vitro , as is the metabolism of a drug if the metabolites themselves are not readily commercially available. There may also be limited information on the full range of active metabolites. Metabolites may have varying half-lives, even existing only transiently, complicating an experiment that is trying to replicate clinical scenarios. The volatility of some chemotherapy agents/metabolites is another consideration, as these can produce “next well” effects, requiring different treatment groups to be physically separated during culture.
Overall, whilst in vitro techniques are valuable, they cannot simply replace in vivo experiments. The clinical scenario will involve indirect effects and potential input from other organs. Thus, in vivo systems are required to determine secondary effects from other organs, including changes to the hypothalamic–pituitary–gonadal axis. Nonetheless, use of in vitro culture systems reduces the number of animal experimentations required and allows for screening of a large range of drugs and drug doses. In particular, high drug dosages can be investigated more easily in vitro , since the experiments are not complicated by deteriorations in animal health or subsequent mortality. Overall, therefore, in vivo and in vitro methods both have their advantages and disadvantages, and both should be used for thorough investigation of reproductive toxicology.
6.3
What Culture Systems are Available?
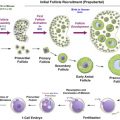
At present, a variety of culture systems are available, using tissue from humans, non-human primates, various domestic species and rodents, and each supporting different stages of follicle development ( Figure 6.1 ). Within the ovarian cortex, the vast majority of follicles are primordial and it is this follicle reserve that dictates the reproductive lifespan of a woman. Therefore, a culture method that can evaluate the potential direct effect of chemotherapy drugs on primordial and early-stage follicles is one of the most clinically relevant systems. The ultimate culture method will support development of oocytes from primordial follicles through to fully mature, fertilizable oocytes in vitro . This is a major goal for reproductive biology, particularly with regards to human fertility preservation, but which to date has been achieved only in the mouse.
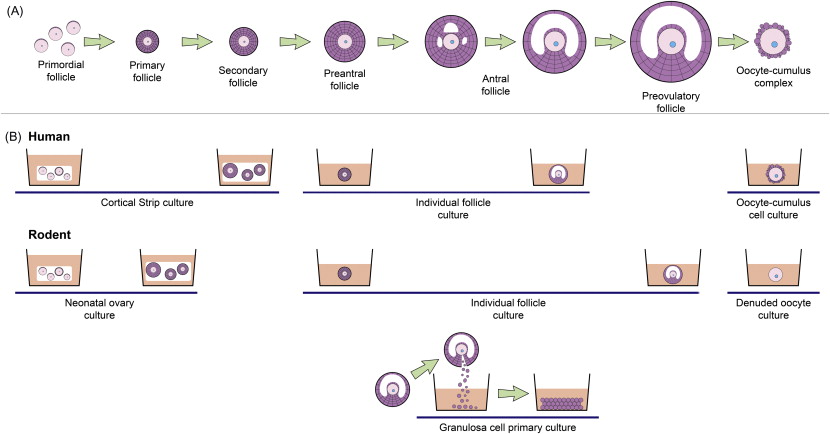
Primordial follicles do not survive culture well when isolated from ovarian tissue, most likely due to their small size and to the removal of support from the surrounding stroma and dense extracellular matrix. In vitro systems need to support the growth and activation of these follicles, which can be achieved through culturing cortical fragments (e.g., primates and ruminants) or culturing whole neonatal ovaries (e.g., rodents). Fragmentation of ovaries is also an increasingly utilized culture technique, which in rodents appears to promote follicle growth.
At present, development of germ cells from primordial follicles through to the production of live offspring has been achieved only in the mouse. In humans, whilst great progress has been made in this direction, culture systems are yet to be fully developed to the point where they can be utilized clinically. Since offspring have not been obtained from starting material of primordial follicles outside of the mouse, it is, at this point, unclear for other species quite how closely physiological conditions have been replicated in vitro . Current experimental protocols aiming at long-term follicle development using ovaries from large mammals usually utilize one of two culture techniques. One approach is to use a multi-step culture system, which involves cortical strip culture to support primordial follicle activation followed by microdissection and individual follicle culture to the antral stage : the final stage involves removing the oocyte–cumulus cells from the antral follicle and in vitro maturation of the oocyte followed by fertilization. Alternatively, immature follicles are cultured within a support matrix such as alginate hydrogels; to date, success has been achieved in culturing non-human primate individual secondary follicles contained in alginate to the antral stage.
6.4
Human and other Primate Model Studies of Ovarian Chemotherapy Toxicity
Human ovarian tissue for culture is usually obtained from one of three sources: fetal ovaries following termination of pregnancy; biopsies from women undergoing caesarean section/gynaecological surgery; or from fertility-preservation procedures. Depending on the age of the tissue at the time of collection, fetal ovaries may have germ cells that have not yet formed follicles. Furthermore, the immature tissue could potentially respond differently to insult than a mature ovary. Thus, fetal tissue is not the most appropriate model in which to investigate chemotherapy-induced toxicity in adults, although it is of course necessary to study effects of in utero exposure. Tissue supply from women of the most clinically relevant age group is often limited, with the most desirable source being biopsies from young women, likely to have ovaries containing a relatively high density of follicles. As patient age increases, total follicle number declines, making it less likely that a biopsy will contain follicles for analysis. In addition, ovarian biopsies from older women usually have a high heterogeneity of follicle distribution, making it difficult to predict whether a specific piece of tissue contains follicles and making it hard for follicles to be evenly divided across treatment groups (if even present at all). One potential way around this is the use of vital dyes such as neutral red to identify the presence of follicles within slices of cortex before culture.
Due to the limited quantity of human tissue available for culture, an alternative is to culture material from non-human primates as a closely related model. Whilst this has been more commonly used in other studies investigating follicle development, to the best of the authors’ knowledge this has only very recently been deployed for the testing of chemotherapy-induced toxicity, in a study culturing slices of marmoset ovary. Non-human primate ovary culture is a relatively new model, but could potentially be very useful in this regard, as it is the closest available species model to humans, with non-human primates having, for example, a similar protracted period of folliculogenesis in vivo . Domestic species and ruminants are another alternative to the use of human tissue, but again, to the best of the authors’ knowledge, they have not yet been used to test chemotherapy ovotoxicity.
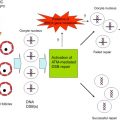
For chemotherapy studies, culturing human ovarian tissue is clearly advantageous, eliminating concern about potential species differences that could exist in the tissue’s response to chemotherapy treatment. However, very few studies have been conducted to investigate chemotherapy-induced toxicity in vitro using human tissue. As discussed above, the ideal model system will support primordial follicle growth, as this best reflects the ovarian reserve. The isolation of small follicles from human ovaries is difficult due to the density and toughness of the stromal tissue, and so culturing small slices of cortical tissue bypasses this issue and allows for the rapid activation of follicles. In cortical strips exposed to high concentrations of cyclophosphamide in vitro , primordial and early-stage follicles exhibited increased vacuolization and damage to granulosa cell nuclei and follicle basement membranes, as assessed by electron microscopy. Doxorubicin increased apoptosis in primordial follicles and stromal cells along with damage to the microvasculature in cultured human cortical strips. Specifically, doxorubicin induced DNA double-strand breaks in both human and marmoset ovary culture studies; the primate model was used further to investigate ovarian protection against chemotherapy-induced damage, showing the ability of dexrazoxane to help prevent this damage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree