Implications of Obesity in Breast Cancer
Ayca Gucalp
Patrick G. Morris
Clifford A. Hudis
Andrew J. Dannenberg
INTRODUCTION
Obesity is a major risk factor for the development of some of the leading preventable causes of death in the United States including cardiovascular disease, diabetes mellitus, and stroke. In addition, epidemiologic studies link obesity with an increased incidence of common epithelial malignancies including postmenopausal hormone-receptor-positive and triple-negative breast cancers (TNBC). Obesity is also recognized as a poor prognostic factor among survivors of breast cancer irrespective of menopausal status and breast cancer subtype. The underlying mechanisms for these phenomena remain incompletely understood. Growing evidence suggests that complex interactions between multiple pathways regulating estrogen synthesis, insulin resistance, adipokine and cytokine production, and chronic systemic inflammation may collectively explain the link between obesity and breast cancer pathogenesis. Increasing adiposity also poses additional technical challenges in the detection of breast cancer and in its effective local and systemic treatment. This chapter reviews the epidemiologic data linking obesity and breast cancer, the underlying biological mechanisms, and the important practical implications of this condition on the diagnosis and management of this disease. Finally, we examine potential interventions and strategies to reduce the unfavorable impact of obesity on the diagnosis and treatment of breast cancer.
EPIDEMIOLOGY OF OBESITY
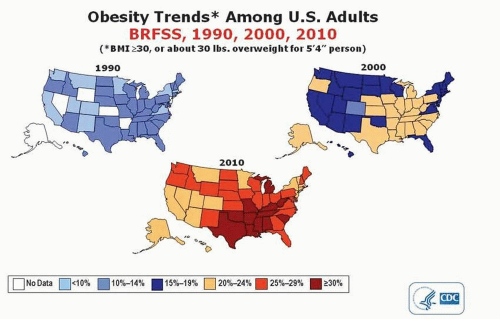
Obesity has been classified by the World Health Organization as a global epidemic (1). The number of overweight and obese individuals in the United States has doubled since 1990, and it is projected that the prevalence of obesity will be greater than 50% in 39 states by the year 2030 (Fig. 49-1) (2).
One commonly used measure of extent of body fat is body mass index (BMI), calculated by dividing an individual’s weight in kilograms by the square of his or her height in meters (1). For adults, BMI is categorized into the following four standard groups: underweight less than 18.5 kg/m2, normal 18.5 to 24.9 kg/m2, overweight 25.0 to 29.9 kg/m2, and obese 30 kg/m2 or more (1). Although BMI is reproducible, inexpensive, and easy to measure and calculate, it is nonetheless only a surrogate measure of body fat. The correlation between BMI and percentage of body fat varies by age, ethnicity, gender, and muscle mass. For example, BMI routinely overestimates the adiposity of trained athletes and underestimates the true percentage of body fat of older individuals with less lean muscle mass (1).
EPIDEMIOLOGIC LINK BETWEEN OBESITY AND BREAST CANCER
Postmenopause
Many observational studies have demonstrated that increasing BMI is a risk factor for postmenopausal hormone-receptorpositive breast cancer. This is the most common subtype of breast cancer. Of note, breast cancers are considered “hormone sensitive” when they expresses the estrogen receptor (ER) and/or progesterone receptor (PR). However, gene expression profiling has allowed us to subdivide hormone-receptorpositive breast cancers into those that are likely sensitive to hormone therapies (“luminal A”) and those that are usually not (“luminal B”). Hence, hormone receptor status is an imperfect surrogate for hormone sensitivity. Of course, the absence of these receptors is very predictive of hormone insensitivity. In a meta-analysis of eight prospective cohort studies, increasing BMI was directly correlated with breast cancer risk in postmenopausal women (3). Compared to those with a BMI less than 22.5 kg/m2, women with a BMI in the overweight to obese range had an increased relative risk (RR) of 1.36 to 1.62 of developing breast cancer (Table 49-1) (3).
Premenopause
The effect of overweight and obesity on breast cancer risk in premenopausal women is less clearly defined. More precisely, it appears likely that the influence of weight may differ based on an individual patient’s underlying risk. Cecchini et al. reported the pooled analysis of two large, randomized breast cancer prevention trials (Breast Cancer Prevention Trial [P1] and the Study of Tamoxifen and Raloxifene [STAR]), which included nearly 32,000 women at high risk of developing the disease. In comparison to premenopausal women with normal BMI, overweight (hazard ratio [HR] 1.59, 95% CI 1.05-2.42) and obese women (HR 1.70, 95% CI 1.10-2.63) had an increased risk of developing invasive breast cancer (4).
Several studies have also suggested an association between adult obesity and the development of TNBC, which lacks expression of the ER, PR, and human epidermal growth factor receptor 2 (HER2). In the Women’s Health Initiative (WHI), a longitudinal study of postmenopausal women, in which over 5,000 invasive breast cancers were diagnosed, the highest quartile of BMI (31.5 or higher) was associated with a 1.39-fold (95% CI 1.22-1.58) increase in the risk of ER-positive breast cancer when compared to women with a BMI in the lowest quartile (less than 23.75) but a similar magnitude of risk of TNBC was also seen (HR 1.35, 95% CI 0.92-1.99), although this latter result was not statistically significant (5).
Impact on Treatment Outcomes
Studies examining the prognostic effect of overweight or obesity after a breast cancer diagnosis have consistently demonstrated poorer clinical outcomes for women with
an elevated BMI. In a recent meta-analysis, Protani and colleagues examined 43 studies with sample sizes ranging from 100 to more than 420,000, and included women diagnosed with breast cancer from 1963 to 2005. The authors demonstrated that obese women consistently experienced higher breast cancer-specific (HR 1.33, 95% CI 1.19-1.50) and allcause mortality (HR 1.33, 95% CI 1.21-1.47) in comparison to their nonobese counterparts (6). Obesity has also been associated with several other poor clinical outcomes including increased risk of contralateral breast cancer and second primary breast cancers (7).
an elevated BMI. In a recent meta-analysis, Protani and colleagues examined 43 studies with sample sizes ranging from 100 to more than 420,000, and included women diagnosed with breast cancer from 1963 to 2005. The authors demonstrated that obese women consistently experienced higher breast cancer-specific (HR 1.33, 95% CI 1.19-1.50) and allcause mortality (HR 1.33, 95% CI 1.21-1.47) in comparison to their nonobese counterparts (6). Obesity has also been associated with several other poor clinical outcomes including increased risk of contralateral breast cancer and second primary breast cancers (7).
TABLE 49-1 Relative Risk of Breast Cancer Development by Body Mass Index | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
OBESITY-RELATED PATHWAYS AND BREAST CANCER PATHOGENESIS
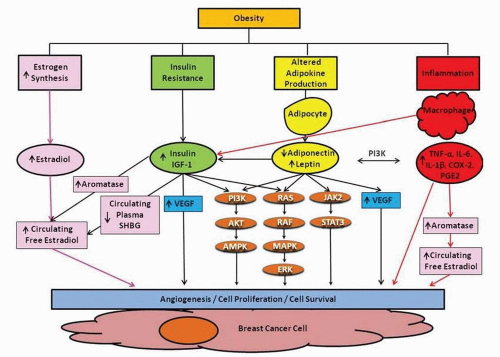
Several lines of evidence suggest that obesity-induced tumor development, invasion, and progression are mediated by a complex interplay between both estrogen dependent and independent pathways (Fig. 49-2). Following the decline of ovarian function at menopause, estrogen production predominantly occurs by the peripheral conversion of androgen precursors to estrogens in extragonadal sites such as adipose tissue. The rate-limiting step in this process is catalyzed by the enzyme aromatase, encoded by the CYP19 gene. Breast cancer incidence increases with age and it is in the setting of lowered circulating estrogen levels that the highest rates of hormone sensitive breast cancer are diagnosed. This seemingly paradoxical phenomenon is thought to be partially due to two related factors: increased adipose tissue and elevated aromatase expression in adipose tissue (8).
In addition to peripheral fat stores, the majority of the normal female breast is composed of white adipose tissue (WAT). WAT is an active endocrine organ producing various hormones, growth factors, and cytokines. Complex interactions between adipocytes and immune cells, such as activated macrophages, have been shown to promote chronic inflammation in the tumor microenvironment as well as insulin resistance. Through multiple interactive pathways involved in energy metabolism and immune function, adipose tissue is thought to contribute to breast cancer development and progression (Fig. 49-2).
Insulin Resistance
Obesity is associated with insulin resistance, characterized by impaired glucose tolerance and elevated systemic levels of insulin and insulin-like growth factor-1 (IGF-1), which have both been associated with breast cancer risk and worse prognosis (9). In a cohort of 512 patients with early-stage breast cancer, elevated fasting insulin levels were associated with both distant recurrence (HR 2.0, 95%
CI 1.2-3.3) and all-cause mortality (HR 3.1, 95% CI 1.7-5.7) (9). It is theorized that insulin mediates breast carcinogenesis by stimulating signaling through activation of insulin/IGF-1 receptors that can be overexpressed on human breast cancer cells. Downstream activation of the Ras-Raf-MAPK and PI3K/Akt pathways ultimately leads to tumor cell proliferation and inhibition of apoptosis. Additionally, IGF-1 and insulin have been shown to stimulate aromatase activity in adipose tissue. Moreover, increasing insulin levels can affect sex hormone biosynthesis leading to increased androgen production by the ovaries and decreased hepatic production of sex hormone-binding globulin (SHBG) that normally antagonizes some negative effects of circulating estrogens.
CI 1.2-3.3) and all-cause mortality (HR 3.1, 95% CI 1.7-5.7) (9). It is theorized that insulin mediates breast carcinogenesis by stimulating signaling through activation of insulin/IGF-1 receptors that can be overexpressed on human breast cancer cells. Downstream activation of the Ras-Raf-MAPK and PI3K/Akt pathways ultimately leads to tumor cell proliferation and inhibition of apoptosis. Additionally, IGF-1 and insulin have been shown to stimulate aromatase activity in adipose tissue. Moreover, increasing insulin levels can affect sex hormone biosynthesis leading to increased androgen production by the ovaries and decreased hepatic production of sex hormone-binding globulin (SHBG) that normally antagonizes some negative effects of circulating estrogens.
Altered Adipokine Production
Adipokines, a group of proteins synthesized and secreted from adipose tissue, are also implicated in the interaction between obesity and breast cancer. Decreased serum adiponectin and elevated leptin levels have been associated with obesity and type 2 diabetes mellitus. Similar disturbances in these adipokines have also been associated with increased breast cancer risk (10) and worse prognosis (11). Although the mechanisms underlying these phenomena are incompletely understood, adiponectin is thought to exert its effects through the activation of multiple downstream signaling pathways including AMPK, PI3K/mTOR, and the transcription factor, nuclear factor-kappaB (NF-κB), which have also been implicated in breast cancer development (12). In addition, adiponectin has been shown to inhibit the growth of multiple breast cancer cell lines (13).
In contrast, leptin is thought to affect different aspects of breast tumorigenesis such as cell growth, angiogenesis, and metastasis and is to known to act mainly through activation of the JAK/STAT, MAPK/ERK, and PI3K/Akt signaling pathways leading to increased cell migration, invasion, and cell survival. Leptin-induced proliferation has been associated with overexpression of c-myc and cyclin D1which are important in cell cycle regulation (12). Finally, leptin has also been reported to have both proinflammatory and angiogenic properties, which may be important for breast carcinogenesis.
Obesity-Induced Chronic Systemic Inflammation: The Obesity-Inflammation-Aromatase Axis
Obesity causes chronic subclinical inflammation in adipose tissue (14). In obese women, increased levels of proinflammatory mediators such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) are commonly found in the circulation and may contribute to breast cancer progression and mortality. Interactions between adipocytes and immune cells promote this proinflammatory response through tolllike receptor- (TLR)-mediated signaling pathways and the activation of NF-κB. In mouse models of obesity and in overweight and obese humans, macrophages infiltrate visceral and subcutaneous adipose tissue and form characteristic crown-like structures (CLS) around dead adipocytes. In both dietary and genetic models of obesity, CLS occur in the adipose tissue of the mouse mammary gland as well as in visceral fat (15). Similarly, in women undergoing mastectomy, the presence of CLS of the breast (CLS-B) was associated with increased BMI. Importantly, the presence of CLS-B is associated with NF-κB-dependent upregulation of multiple proinflammatory mediators such as interleukin-1β (IL-1β), cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and TNF-α, which leads to increased transcription of CYP19, the gene encoding aromatase (16, 17). These elevated levels of proinflammatory molecules and aromatase expression and activity are paralleled by upregulation of PR, an ER target gene. Aromatase activity correlates more strongly with the CLS-B index (p = .88, p < .001), a marker of extent of inflammation, than with BMI (p = .5, p = .02). This finding underscores the fact that obesity-related inflammatory mediators are critical for the induction of aromatase. Collectively, these results establish the existence of an obesity-inflammation-aromatase axis in breast tissue, which is likely to be one mechanism by which obesity influences breast carcinogenesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree