Fig. 24.1
Necrosis and apoptosis zones after cryoablation. Areas closest to the center of the cryolesion reach lower temperatures with higher freezing rates resulting in rapid intracellular ice crystal formation, cell bursting, and necrosis (direct injury). At longer distances from the cryoprobe, only milder freezing temperatures are reached, resulting in a transition from necrosis to apoptosis. The lower freezing rate at distant sites results in intracellular osmolar dehydration, necrosis secondary to extracellular ice crystal formation, or enzymatic changes that result in apoptosis
The direct cell damage described above results in necrosis and release of intracellular contents, as well as extracellular matrix damage, all leading to activation of the short-lived, nonspecific innate immune response and inflammation. The direct mechanical injury resulting in cell necrosis is typical at short distances from the center of the cryogenic lesion given the lower temperatures achieved. Temperatures of approximately −20 °C have been suggested for adequate tissue destruction; however, it is worthwhile to note that malignant cells may be more resistant to cryoinjury and require lower temperatures [16]. Human primary prostatic adenocarcinoma cell lines cryoablated at two different cooling rates of 5 or 25 °C/s required temperatures lower than −40 and −19 °C, respectively, for complete destruction of cells [17]. Other groups have also suggested that tissue temperatures should at least approach −40 °C in tumors for effective ablation of malignant tissue [15, 16]. When human colon carcinoma cells were exposed to a temperature range of −6 to −36 °C, approximately 98.5 % of cells that reached −36 °C were dead by direct cryolysis after 4 h, while only approximately 50 % underwent cryolysis if they only reached −10 °C. Current standards for cryoablation of malignant tumors recommend −50 to −60 °C degrees.
Within higher, sublethal temperature ranges, cryoablated cells in malignant tissues such as in human colon carcinomas have been shown to undergo apoptosis instead of necrosis [18]. Markers for apoptosis can be observed in cells 4 h after freezing. The percent of apoptotic colon carcinoma cells is maximized at −25 °C with approximately 28 % of cells demonstrating elevated apoptosis markers. Colder temperatures result in lower apoptotic rates likely secondary to a shift toward the mechanism of cell injury described above, namely, a predominantly cryolytic type of cell death and tissue necrosis (Fig. 24.1). It is important to note that cell death by apoptosis is the primary mechanism of cryoablation in the peripheral zone of the cryogenic lesion (away from the center of cryolesion), given the inherent higher temperatures found away from the source of freezing. Also notable is that apoptotic cell death does not induce an inflammatory response as seen during necrosis. The balance between apoptosis and necrosis may play an important role in whether a tumor undergoing cryoablation is destroyed or not.
Indirect cell damage also occurs after cryoablation. Ice crystals form in blood vessels damaging the lining endothelium. After thawing and reperfusion of affected vessels, platelets become activated causing thrombus formation and subsequent local tissue ischemia [19]. The vascular stasis results in further tissue damage post-cryoablation. Furthermore, indirect damage occurs due to immunological injury that follows an initial inflammatory response (Table 24.1). This process of immune system activation is described by cryoimmunology and is discussed in the following sections.
Table 24.1
Mechanisms of cell death and tissue injury after cryosurgery
Direct injury | Indirect injury |
|---|---|
Extracellular matrixice crystal formation | Thrombus formation |
Intracellular osmoticdehydration | Vascular stasis and ischemia |
Enzymaticconformational changes | Innate and adaptive immunesystem-driven injury |
Intracellular ice crystalformation and cell bursting |
Inflammation and Immune System Activation After Cryosurgery: The Cornerstone of Cryoimmunology
Cryoimmunology can be described as a process involving inflammation and immune attack against damaged target tissue that follows local cryoablation. As described above, antigens are released after cryosurgery secondary to membrane disruption and cell death. In addition, preformed inflammatory cytokines are also released from the surrounding extracellular matrix, initiating an immediate inflammatory response. For instance, studies have demonstrated that levels of IL-1β, IL-6, and TNF-α are elevated after cryoablation of large hepatic metastases [20, 21]. IL-12 and IFN-γ have also been detected shortly after freezing [1]. Prostate tumor cell gene expression of several cytokines implicated in stimulating immunity, such as IL-2, IL-15, IL-12p40, IL-18, and TNF-α, was demonstrated to increase after cryoablation. Cells including neutrophils, macrophages, and lymphocytes are recruited by the ongoing inflammatory process and add to the cytokine milieu by secreting other inflammatory mediators marking the activation of the innate immune response [22–24]. Cell infiltration including APCs and T cells may be mediated by the expression of proteins such as heat-shock protein 70 (HSP 70) from tumors undergoing cell death [25]. As quickly as within a few hours after cryosurgery, polymorphonuclear (PMN) leukocytes are also recruited to the lesion site and infiltrate the area. Their migration peaks after 3 days and occurs along with an increasing macrophage infiltration that peaks at 7 days [26]. NK cell activity was also shown to increase in a mammary adenocarcinoma mouse model after cryoablation [1].
Following innate immunity activation, the acquired immune response begins with the actions of antigen-presenting cells (APCs). APCs, namely, macrophages and dendritic cells (DCs), engulf the antigens released by cryoablation and present them to T cells via major histocompatibility complex (MHC) classes I and II. A cytotoxic and humoral response develops with APC presentation of antigen to lymphocytic cells. Once antigens are endocytosed by DCs, these cells migrate to lymph nodes where they undergo maturation, becoming efficient APCs able to prime naïve antigen specific T cells. In response to inflammatory and antigenic signals, DCs that have been loaded with endocytosed cryotreated tumor cell fragments upregulate their maturation gene expression including IL-1, Fas, CDKN1A, osteoprotegerin, and CXCR4, as well as their expression of immunostimulatory cytokines including IL-1β, IL-12p40, IL-15, TNF-α, and IFN-γ. DC maturation estimated by flow cytometric measurement of the surface marker CD86, a ligand that participates in T-cell priming, was shown to increase 8-fold after freezing prostate cancer cells [27]. Moreover, the expression of class-II MHC, which is a required component of antigen presentation and activation of T cells, was doubled.
As APCs present antigens to naïve helper T cells (Th) in the presence of IL-12, T-cell differentiation into Th1 cells occurs. Th1 cells release IL-2, IL-12, IFN-γ, TNF-α, and GM-CSF, which in turn participate in activating cytotoxic (CD8) T cells. B-cell proliferation and differentiation into antibody-producing cells also occur upon expression of IL-4 by naïve helper T cells, resulting in T-cell differentiation into Th2 cells. Th2 cells express IL-4 and IL-5 cytokines, promoting B-cell differentiation into antibody-producing cells. The above-described cascade of events marks the initiation of the adaptive arm of the immune response triggered by cryoablation. This response is specific against the antigens derived from cryoablated cells, possibly priming systemic antitumor activity (Fig. 24.2).
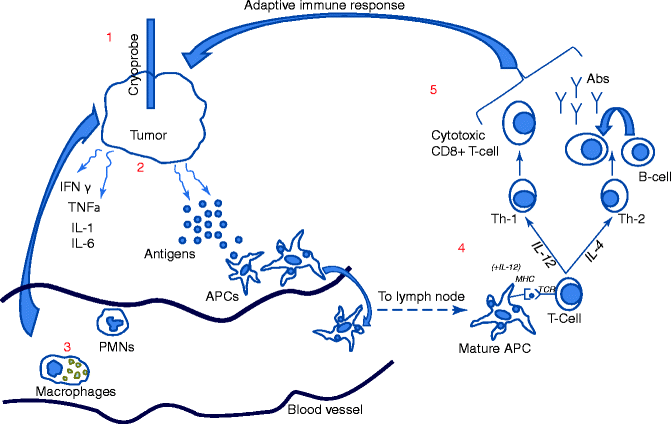

Fig. 24.2
Inflammation and immune system activation after cryoablation. (1) Cryoprobe freezes tumor and associated stroma resulting in (2) the release of antigens and inflammatory cytokines (i.e., IFN-gamma, TNF-alpha, IL-1, IL-6) from tumor and extracellular matrix. (3) Innate immune cells such as macrophages and polymorphonuclear cells (PMNs) migrate to cryolesion in response to chemotaxis caused by inflammatory cytokines and contribute to the inflammatory milieu. (4) Antigen-presenting cells (APCs) including dendritic cells and macrophages endocytose antigens, migrate to lymph nodes where they mature, and present antigens to naïve T cells in the presence of cytokines such as IL-12. (5) T cells differentiate into Th1 or Th2 cells in the presence of IL-12 or IL-4, respectively. Th1 cells undergo differentiation into cytotoxic T cells, whereas Th2 cells contribute to the differentiation of B cells into antibody producing cells marking the initiation of the adaptive immune response
Immunity vs. Tumor Microenvironment
Tumor survival and progression are partly due to their ability to evade the host immune system. Tumor microenvironments appear to modulate immune response by regulating immune cell phenotype and function both locally and systemically. For instance, patients with progressing hepatocellular carcinoma (HCC) have been demonstrated to have increasing levels of peripheral CD4+CD25+ cells, detected by flow cytometry [28]. CD4+CD25+ cells are some of the best-described regulatory T cells (Treg) that downregulate the immune system. Increases in Treg cell levels have also been shown in other cancers including gastric, lung, pancreatic, and breast cancers [29]. It has been speculated that the response to cryoablation may vary depending on tumor type and the level of Treg cells present. For instance, patients’ HCC response after cryoablation with argon-helium cryoprobes to freezing temperatures of −135 °C appeared to be dependent on to the quantities of circulating CD4+Cd25+ Treg cells prior to treatment. Patients with HCC regression after cryoablation showed significantly decreased Treg frequency by flow cytometry. In contrast, patients with HCC progression and recurrence showed increased Treg cells. Treg frequencies were approximately 6.9 % in early stage HCC, 8.8 % in intermediate stage, 10.3 % in advanced stage, and 13.4 % in terminal stage HCC. These results indicate the role of immune downregulation in tumor survival, even after therapy. These data may also help explain why cryoablation is at times ineffective in treating certain tumors. It is likely that adjunctive therapy may be necessary to obtain the full potential antitumor effects of cryotherapy.
Other studies have suggested that cryotherapy may counteract the tumor microenvironment anti-immune activity. The immunological response to cryoablation in a renal cell cancer animal model demonstrated higher IFN-γ to IL-4 levels, suggesting an increase in Th1:Th2 ratio and demonstrating the interplay between inflammatory cytokines and activation of the immune system after cryosurgery. The increase in Th1 to Th2 ratio opposes the anti-immune response effects of renal cell carcinoma (RCC) previously shown [30].
Sabel and colleagues have elegantly demonstrated how rate of freeze could affect the resulting immune response after cryoablation of 4T1 breast carcinoma in BALB/c mice [31]. Two weeks after inoculation of mice with the tumor cells, the animals were divided in four groups (control, surgical resection group, and low- and high-freeze group). High-freeze technique used argon cryoprobe for 30s and duty cycle of 100 %, while low-freeze used the same probe, but with duty cycle of 10 % for several minutes. One week later, high-freeze group demonstrated significant reduction in CD4+CD25+ T-regulatory cells in examined tumor lymph nodes, while the number of cytotoxic CD8+ cells was not significantly affected. Both, low-freeze group and surgery group had dramatically higher Treg levels, producing more immunosuppression and increased levels of lung metastases in the latter. Furthermore, the cytotoxic CD8 T-cell responses to exposure to tumor cells in vitro were almost tripled in the high-freeze group in comparison to low-freeze, surgery, and control. Specific evidence of antitumor activity following cryoablation is discussed in more detail in the following section.
Primary and Metastatic Antitumor Activity
The formation of autoantibodies against cryoablated tissues was demonstrated many decades ago. Early experiments with cryoablation of the rabbit benign prostate, seminal vesicle, and bulbourethral glands showed that autoantibodies specific to these tissues could be isolated from the serum of respective animals. These antibodies did not react with other autologous tissues such as the kidney, liver, or thyroid and did not recognize human-derived tissues, demonstrating the specificity of the antibodies for the cryoablated tissue [32]. Autoantibody formation was detected as early as within 7–10 days of cryoablation. These data led to subsequent studies exploring the generation of immune system-targeted therapy against malignant tissues with cryosurgery. Treatment of prostate cancer with cryoablative techniques has been described more extensively and has shed light into the widespread potential applications of cryoimmunology as primary or adjuvant therapy for cancer. Similar to the above-mentioned investigations with benign prostates, studies performing cryoablation of malignancy-laden prostates demonstrated that serum antibodies specific to prostate cancer antigens were found in the serum after cryosurgery [33]. Interestingly, despite increased antibody levels, actual secondary tumor growth and tumor histology were unchanged. Several other reports have suggested similar contradictory data in regard to the actual efficacy of tumor regression after cryotherapy and are discussed in more detail in a separate section below.
Despite some of the skepticism and contradiction in the data, other investigators have demonstrated impressive benefits of cryotherapy for cancer treatment in human patients. A retrospective study comparing the effects of targeted cryoablation of prostate cancer and control therapies including brachytherapy, external beam radiation, or 3-dimensional conformal radiation demonstrated that cryotherapy resulted in equal or superior disease-free rates [34]. The mean follow-up time was 5.3 years and the outcome measures were prostate-specific antigen (PSA)-based cutoffs. No serious complications were reported and only minimal morbidity was observed.
Patients with nonmetastatic prostate cancer have also been treated using a percutaneous approach to cryoablation [35]. Biopsy of treated tissue after percutaneous cryotherapy showed elevated levels of TNF-α and IFN-γ as well as increased Th1:Th2 ratio. A significant increase in peripheral blood mononuclear cells was also shown along with increased cytolytic activity of cytotoxic T lymphocytes against autologous prostate cancer cells. This activity was specific to prostate tissue. It is prudent to note, however, that despite evidence of immune system activation, this approach was not sufficient to prevent cancer relapse. Moreover, the level of differentiation of the prostate tumor cells also plays a role in the effectiveness of cryosurgery. More differentiated tissue proved to be associated with advantageous survival in patients [36], since the capacity for proliferation decreases in cells as they mature and differentiate.
Some of the rationale for the limited effectiveness of cryotherapy in eradicating tumors may be explained by the expression of inhibitory T-cell coreceptor CTLA-4. CTLA-4 binds B-7 at the surface of APCs. Their interaction inhibits T-cell activation by reducing the expression of IL-2. CTLA-4 is the target of a recently FDA-approved antibody drug, ipilimumab, which can diminish this immune inhibitory process and allow a more robust response. Given the potential to enhance the antitumor effects of thermal ablation, the adjuvant use of ipilimumab along with cryotherapy was demonstrated in a recent study [37]. A mouse model of primary prostate cancer treated with combination of cryoablation and ipilimumab was utilized, showing that the growth of secondary tumors seeded at distant sites after initial thermal ablation of the primary tumor (also known as tumor rechallenge) was slowed and tumor rejection was triggered. The secondary tumors were found to be highly infiltrated by CD4+ T cells, as well as CD8+ T cells, and relatively less T-regulatory cells. This cessation in growth was not observed after cryoablation alone. These results are promising and suggest augmentation of antitumor immunity with combination therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree