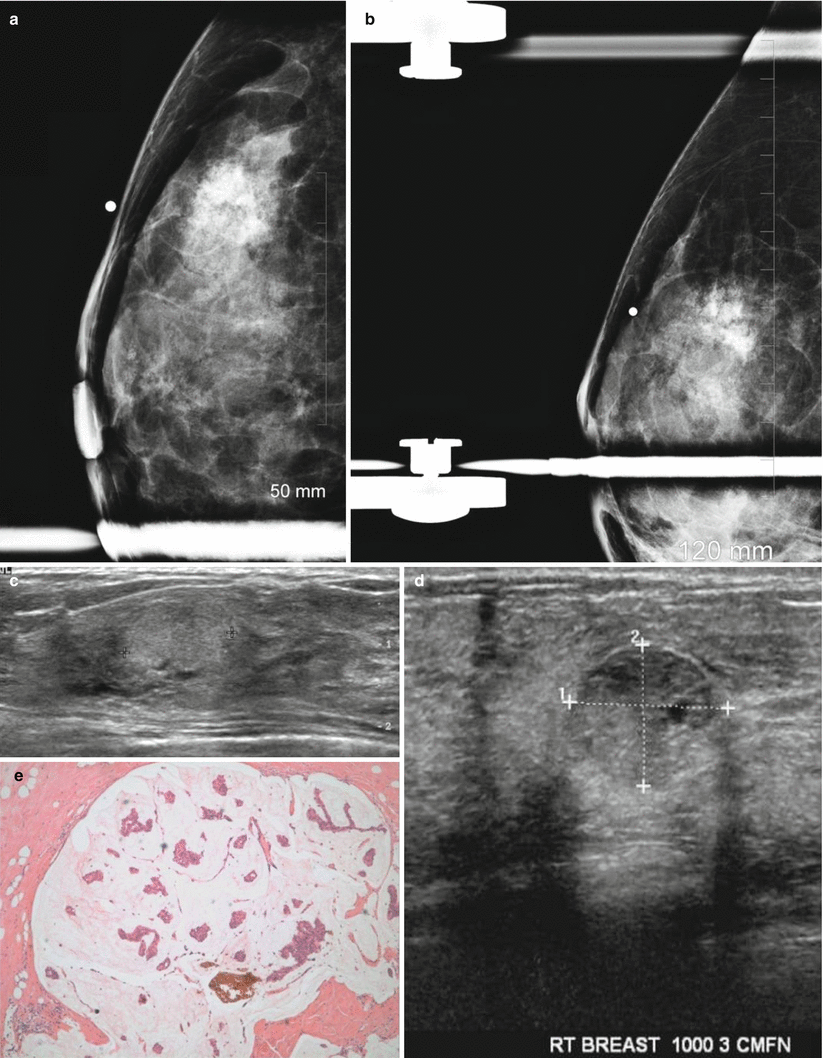
Fig. 13.1
A 35-year-old with a palpable lump histologically proven to be a fibroadenoma. (a) Ultrasound demonstrates an ovoid solid mass with circumscribed borders. (b) Histology demonstrates pericanalicular type of fibroadenoma
Phyllodes Tumor
These are fibroepithelial tumors that present as large tumors and are commonly seen in young women. The average diameter of phyllodes tumor has been reported to be 5 cm, and the median patient age of presentation is 40 years; these masses are frequently palpable [29, 30]. Phyllodes tumors are composed of a hypercellular connective tissue stroma and epithelial elements. Histologically it is possible to distinguish between the benign type and the malignant type, the latter metastasizes in about 25 % of cases. Phyllodes tumor of the breast are locally aggressive tumors. Irrespective of the histological type, these tumors tend to recur after local excision. It is not possible to predict histologically the type that can locally recur. Following local excision nearly half of borderline phyllodes and two-thirds of malignant phyllodes tumors recurred; even following wide local excision 29–36 % of borderline and malignant phyllodes tumors recur [31]. Even the benign type recurs after local excision (21 %) and wide local excision (8 %). It is clear from these data that since patients with benign phyllodes tumor treated with breast-conserving surgery rarely die from their tumor (0.3 %), wide local excision is the preferred procedure for benign phyllodes tumor and mastectomy is the preferred treatment for malignant phyllodes tumor; a recurrence rate of 12 % has been reported even after mastectomy [31]. Phyllodes tumors of the breast are, however, rare tumors of the accounting for less than 1 % of all breast neoplasms [32, 33]. Majority of these tumors are benign particularly in the younger women; about 31 % of phyllodes tumors are borderline or malignant [32, 34]. On mammography, phyllodes tumor appears as a large circumscribed lobulated hyperdense mass, with calcifications rarely associated. On ultrasound these masses are circumscribed and heterogeneously hypoechoic and may have cleft-like or cystic spaces, being indistinguishable from a fibroadenoma [32] (Fig. 13.2a–f). Sonographic differentiation between benign and malignant types is unreliable. A diagnosis of phyllodes on a core needle biopsy is an indication for excisional biopsy. One study found that complex cystic echogenicity, presence of cleft, and a higher final BI-RADSTM assessment were more common in malignant phyllodes tumor [32].
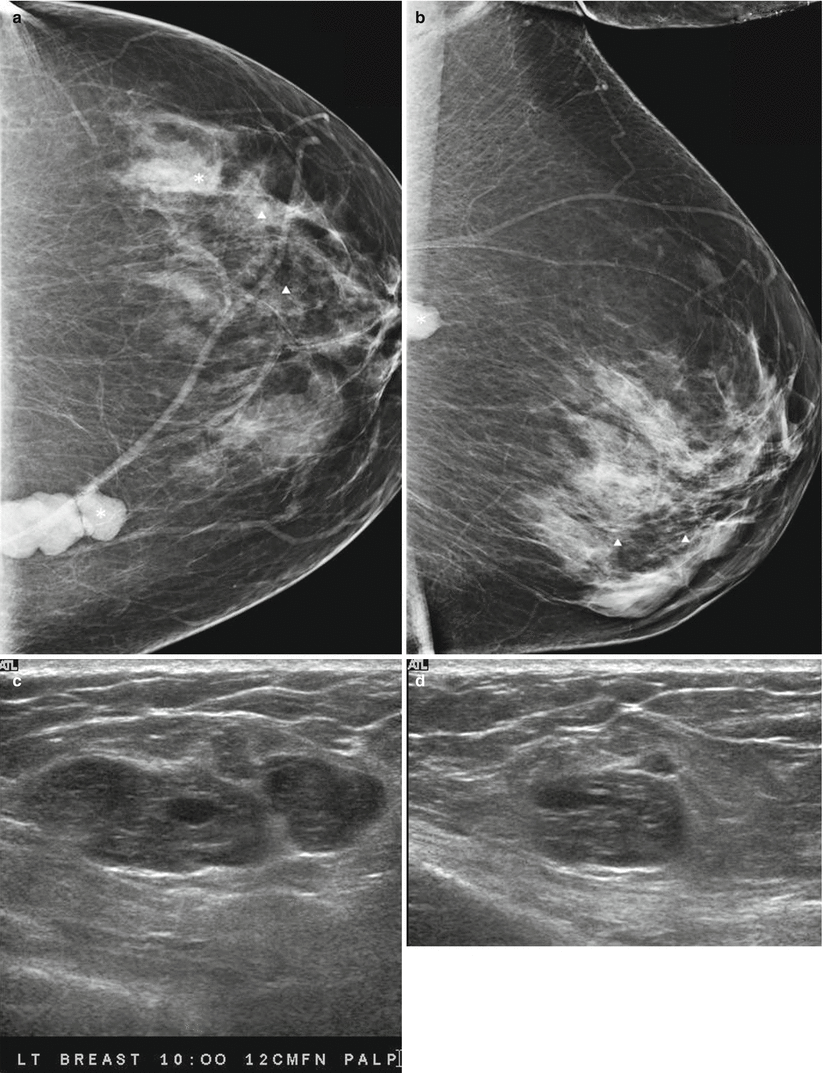
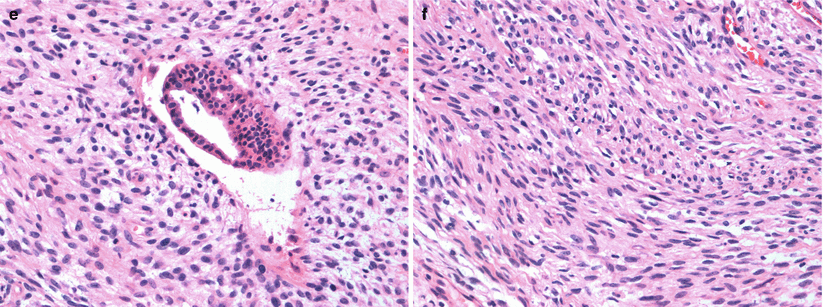


Fig. 13.2
A 55-year-old woman being worked by for excisional biopsy of suspicious calcifications with an incidental mass subsequently proven to be malignant Phyllodes tumor. (a) Craniocaudal view demonstrates a high-density lobulated mass in the inner breast whose posterior margin could not be included on the mammogram. (b) Mediolateral oblique view demonstrates only a part of the mass. (c, d) Ultrasound demonstrates a solid mass with cystic changes and ill-defined borders. (e, f) Histological slides
The treatment of nonmetastatic phyllodes tumors of the breast is complete surgical resection with wide resection margins. Lumpectomy or partial mastectomy is the preferred surgical therapy. Local failure rate is high and 22–25 % of malignant phyllodes tumors metastasize, most frequently to the lungs. Factors that are predictive of an increased risk of recurrence include positive surgical margins, increased stromal cellularity, stromal overgrowth, stromal atypia, and increased mitotic activity [35].
Focal Fibrosis
This entity is also referred to as stromal fibrosis. Histologically focal fibrosis is composed of dense collagenous stroma with sparse glandular and vascular elements [22]. Focal fibrosis has been reported in 2–15 % of breast lesions undergoing tissue diagnosis [36–40]. On a mammogram focal fibrosis appears as a mass or a focal asymmetry; calcifications are rare (Fig. 13.3a, b). Masses are the most common mammographic appearance and have been reported to be seen in 46–75 % of cases [36, 38–40]. Focal asymmetry has been reported in 10–39 % of cases [36, 39, 40]. Architectural as a sign of focal fibrosis is less common and is seen in 5–12 % of cases [36, 39]. The diagnosis of focal fibrosis on core needle biopsy can be considered concordant for a mass exhibiting well-circumscribed or partially obscured margins. Imaging findings discordant with focal fibrosis, such as marginal spiculation, require excisional biopsy [36].
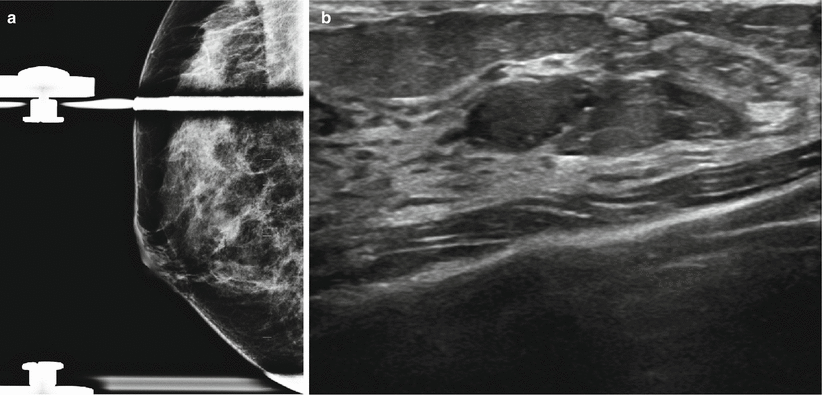

Fig. 13.3
A 47-year-old woman with an abnormality in the right breast histologically proven to be focal fibrosis. (a) Mediolateral oblique view with spot compression demonstrates a focal asymmetry. (b) Ultrasound demonstrates an ovoid solid mass with indeterminate sonographic features
In 10 % of cases these lesions are mammographically occult and are identified on ultrasound [36]. At ultrasound focal fibrosis frequently demonstrates a mass with indeterminate features prompting a biopsy [16, 36–40]. Sonographically, 72 % (n = 36) of cases of focal fibrosis presented as masses with three echotexture patterns: hypoechoic, isoechoic, and centrally echogenic with a peripheral hypoechoic rim. The sonographic margins were well circumscribed (n = 21), lobulated (n = 10), or ill defined (n = 5). Histological review revealed three morphological patterns of collagen deposition: perilobular, septal, and haphazard fibrosis. Correlation with the imaging findings shows that the septal and perilobular fibrosis pattern is most often associated with a hypoechoic or a centrally echogenic mass, whereas the haphazard form of fibrosis is associated with architectural distortion [36]. Calcifications, although considered rare, have been reported in 9 % of cases in one series [40].
PASH: Pseudoangiomatous Hyperplasia
Pseudoangiomatous hyperplasia may be seen in up to 25 % of breast biopsy specimens with a probable hormonal cause in premenopausal women [41]. Histological features are similar to a fibroadenoma with predominant finding of sheets of benign ductal cells interspersed with anastomosing slit-like spaces that are lined by spindle cells unlike vascular channels that are lined by endothelial cells and contain red blood cells [42]. PASH is believed to be hormonally induced and accompanied by benign epithelial proliferation in ducts and lobules [43]. The imaging features in PASH have been reported in several studies [43–46]. Two series of 149 and 73 cases reported that on mammography a noncalcified mass or focal asymmetry described as localized increased stroma was the most common finding [43, 44]. There is no risk of malignancy and a diagnosis of PASH at core needle biopsy should be followed by a routine follow-up. Risk of recurrence is low and about 2 % has been reported [43]. At mammography PASH appears as a circumscribed mass or focal asymmetry (Fig. 13.4a–d). Substantial numbers can be mammographically occult. Architectural distortion and calcifications are rare. Majority of lesions are asymptomatic; however, 29 % of lesions were palpable in one series [44]. On ultrasound it appears as a circumscribed mass without malignant features, and increased through transmission has been reported in 69–81 % of cases [43]. Presence of a suspicious finding such as associated calcifications or spiculated borders should be considered discordant and excisional biopsy is advised since a small percentage of PASH can be associated with invasive cancer.
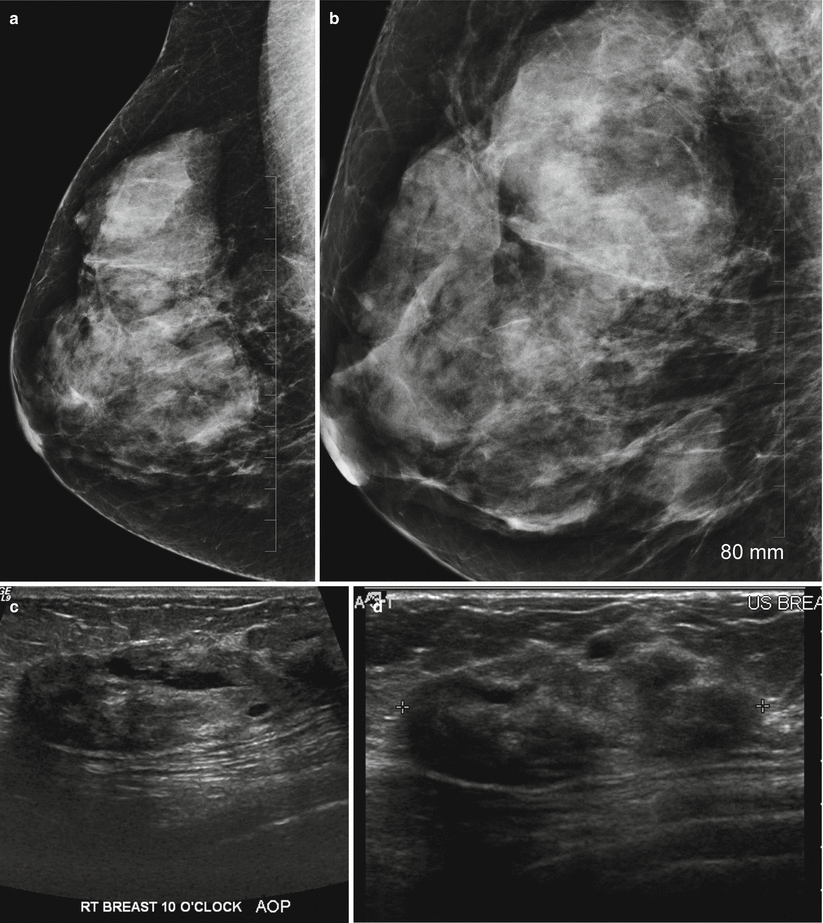

Fig. 13.4
A 53-year-old woman with a palpable mass in the right breast histologically proven to be PASH. (a) Mediolateral oblique view with spot compression shows a large focal asymmetry in the upper posterior part of the right breast. (b) Craniocaudal view demonstrates the focal asymmetry in the outer breast. (c, d) Ultrasound demonstrates an ovoid mass with heterogeneous echotexture and ill-defined margins
Sclerosing Lobular Hyperplasia or Fibroadenomatoid Mastopathy
This is a benign proliferative lesion seen most often in young black females with a mean age presentation of 32 years [22]. A common appearance is that of a circumscribed noncalcified mass resembling a noncalcified fibroadenoma. Histologically it is characterized by enlargement of lobules, increased number of intralobular ductules, and sclerosis of the intralobular septa [47].
Imaging Pathological Correlation in the BI-RADSTM 5 Assessment Category
BI-RADSTM 5 Assessment with Benign Histology
Mammary Fibromatosis (Desmoid Tumor)
Fibromatosis or desmoid tumor of the breast is rare comprising 0.2 % of breast tumors. It is a benign, nonmetastasizing low-grade spindle cell stromal tumor, which usually presents as a spiculated locally invasive tumor that is often palpable. Average age of presentation is 37 years. There is often a history of trauma or surgery. Recurrence rate is about 25 %. Wide excisional biopsy is recommended. Histologically there is profuse collagen and spindle cells. Absence of mitotic figures distinguishes from fibrosarcoma. Mammographically a desmoid tumor appears as a dense irregular noncalcified spiculated mass. On ultrasound an irregular hypoechoic mass with spiculated borders is typical [22, 48, 49] (Fig. 13.5a–d).
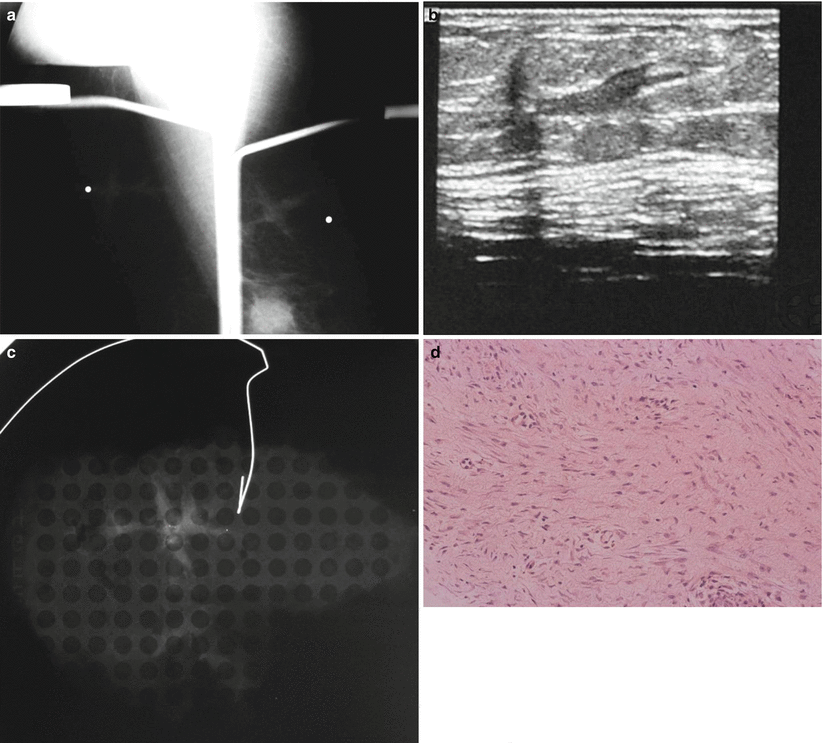

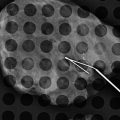
Fig. 13.5
A 46-year-old with a palpable lump in left breast. Excisional biopsy revealed mammary fibromatosis (desmoid tumor). (a) Spot compression magnification views show an irregular mass. (b) Ultrasound demonstrates a hypoechoic solid mass with a branching pattern. (c) Specimen radiograph demonstrates the dense irregular mass. (d) Hematoxylin and eosinophil staining showing interlacing bundles of spindle cells characteristic of a desmoid tumor
Diabetic Mastopathy
Diabetic mastopathy is characterized by stromal proliferation and is found in women with juvenile-onset insulin-dependent diabetes. Breast lesions are seen in about one half of all female patients with type 1 diabetes [22]. Palpable hard nontender fibrous masses that are frequently multiple and bilateral are encountered. Histologically these are collagenous stroma with increased number of spindle cells and scattered epithelial cells and associated lymphocytic infiltrate in the perivascular spaces [50]. There is no associated risk of breast cancer. Eighty-five percent of lesions were palpable in one series [51]. Diabetic mastopathy appears on the mammogram as a mass or focal asymmetry. On ultrasound these masses are hypoechoic and may have irregular margins corresponding to histological findings of poorly circumscribed and irregular margins. Vascular calcifications are present in a substantial number of cases in the excisional specimen suggesting that vascular damage and wound healing process may contribute to the pathogenesis of this entity [51]. Diabetic mastopathy is often seen in premenopausal women with diabetic complications such as retinopathy. MRI is helpful in diagnosing the benign nature of these masses [51]. Histologically there is lymphocytic lobulitis and ductitis with glandular atrophy as well as lymphocytic and mononuclear perivascular inflammation and dense fibrosis with or without epithelioid-like fibroblasts [51]. Fibrous mastopathy may also occur in nondiabetic patients, in those with autoimmune disease, or in healthy subjects. Recurrence may be seen in up to a quarter of cases [52]. Up to 39 % had an ultrasound appearance of malignancy in one series [52]. Fibrous mastopathy may simulate breast carcinoma on clinical examination, mammography, and ultrasound [53].
Radial Scars
Radial scar of the breast is a benign lesion that mimics a cancer on mammography, sonography, and histologically. There are two types of radial scars that one needs to be aware of: incidental microscopic radial scars that are encountered in breast biopsy specimens and the macroscopic ones identified at imaging. The latter when less than 1 cm are referred to as radial scars and when greater than 1 cm is called complex sclerosing lesions. These larger lesions carry a risk of coexisting carcinoma and can be histologically difficult to distinguish from tubular carcinoma. The incidental microscopic radial scars do not carry an increased risk for associated breast cancer [54]. Radial scars are histologically characterized by the presence of a central fibroelastic core containing entrapped glandular elements and radiating ductal elements that gives the lesion a characteristic stellate appearance. Radial scars due to presence of entrapped glands in the central portion can mimic tubular carcinoma; however, they can be differentiated by special stains that identify myoepithelial cells in radial scars [55]. An association of radial scar with breast cancer has been reported in 32–46 % of cases, being higher in symptomatic cases [54, 56]. In the screen-detected group, association with breast cancer has been reported in 8 % of cases. There have been no differences in the rate of associated cancers between radial scars and complex sclerosing lesions [56].
Radial scars of the breast are benign lesions that are usually detected incidentally on screening mammography and are difficult to distinguish from breast cancer on mammography. The typical mammographic appearance of radial scars of the breast has been described as showing an absence of a central opacity often substituted by a radiolucent area, presence of multiple, elongated, thin spicules radiating from the center of the lesion, varying appearance in different projections, and absence of a palpable abnormality (Fig. 13.6a, b). Although reported in up to 28 % of benign breast biopsy specimens, they are much less frequently identified on mammograms. A detection rate of three per 1,000 mammograms was reported in one series [57–60]. Because mammographic appearance is suspicious and because of the known risk of coexisting breast cancer, excisional biopsy is indicated even when the mammographic appearance is suggestive of a radial scar [54–56].
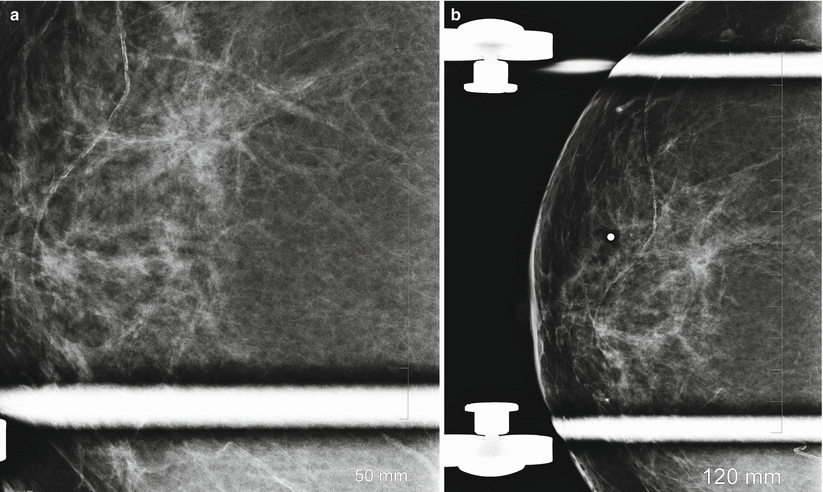

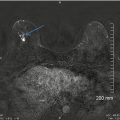
Fig. 13.6
A 57-year-old woman with a histologically proven radial scar in her right breast. (a) Spot compression mediolateral oblique view demonstrates an area of distortion and spicules radiating from an area of decreased density. (b) Spot compression craniocaudal view demonstrates an area of distortion and spicules radiating from an area of decreased density
Sonography is generally considered to have no role in the imaging of lesions mammographically suggestive of a radial scar. However, there have been reports of its value particularly when seen only one view and for guidance of biopsy [57]. On ultrasound a radial scar can have an appearance that is highly suggestive of malignancy or appear as round solid masses or area of shadowing without a focal mass [57]. The incidence of incidental microscopic radial scars in benign breast biopsy specimens has been reported to be 7.1 % [99/1,396] and had a median size of 4 mm [61].
Fat Necrosis
Fat necrosis in the breast is a benign condition that results most commonly as a consequence of iatrogenic trauma to the breast tissue as a result of percutaneous biopsy or breast surgical procedures and uncommonly secondary to anticoagulant therapy or collagen vascular diseases [62]. It may be palpable or incidentally identified on screening mammogram. Fat necrosis can mimic cancer on imaging studies. Histologically it is characterized by destruction of fat cells with hemorrhage and development of vacuoles filled with necrotic lipid material that leads to an inflammatory cell infiltrate with histiocytes that phagocytose necrotic debris within these vacuoles [62–64]. During the next phase of repair fibroblasts proliferate at the periphery of the lesion thereby surrounding areas of fat and necrotic debris. During this phase characteristic calcifications appear. At mammography the spectrum of appearance ranges from the commonly encountered oil cyst that appears as a radiolucent mass that can be confidently categorized as a benign finding to a mass with spiculated borders which in the absence of an area of radiolucency may appear as a suspicious mammographic finding. Presence of coarse dystrophic calcifications are common and can be characterized as benign; however, not uncommonly one may see amorphous or pleomorphic calcifications that may lead to a biopsy recommendation. Ultrasound appearance on the other hand is more often indeterminate and in the absence of correlation with a more frequently associated characterized benign features on mammography may prompt a biopsy.
On ultrasound one may find a sharply demarcated anechoic mass without posterior acoustic enhancement or even posterior acoustic shadowing, an irregular mass with a spiculated border, a complex cystic mass, or an oval or round indeterminate solid mass. The intracystic contents may represent hemorrhage or necrosis and spiculated borders may represent the phase of fibrosis. Less than 2 % of breast biopsies result in a diagnosis of fat necrosis at excisional biopsy [64]. About 27 % of fat necrosis appears as a radiolucent oil cyst; about 12 % appear as a round mass; focal asymmetry has been reported in 16 % of cases. Suspicious findings such as pleomorphic calcifications are seen in less than 4 % of cases. Ultrasound shows solid masses in about 14 % of cases, complex cysts in 11 %, and mural nodules in less than 4 % [65].
High-Risk Lesions: Excisional Biopsy Following CNB
These are lesions that carry a risk of associated cancer that may be underestimated on percutaneous core needle biopsy and hence are managed with excisional biopsy [66, 67] (Box 13.1). In this group we have the radial scar, atypical ductal hyperplasia, benign papilloma, lobular neoplasia, flat epithelial atypia, and mucocele-like lesion. Most of the studies that have examined the risk of an upgrade to malignancy have design flaws which make it difficult to draw conclusions on the real risks [66]. These limitations include lack of data on follow-up of all lesions that are not surgically excised or inclusion of cases with imaging pathological discordance [66]. These are retrospective studies that are also limited by the small number of cases studied since these high-risk lesions are small in number and statistical significance is hard to establish [66].
Box 13.1. Histology at Core Needle Biopsy Where Excisional Biopsy Is Generally Indicated
Atypical ductal hyperplasia |
Papillary lesions |
Radial scars |
Flat epithelial atypia |
Atypical lobular Hyperplasia |
Mucocele-like lesions of the breast |
Lobular carcinoma in situ |
Radial Scars
Due to the risk of missing an associated cancer and the difficulty in distinguishing it from a tubular cancer, all cases of radial scar found on percutaneous biopsy are generally recommended to undergo excisional biopsy. The rate of associated cancer is between 5 and 9 % with a lower rate when larger 11-g needle samples are obtained [68, 69]. This rate is not reduced in radial scars without atypia, and hence irrespective of associated atypia, all radial scars diagnosed at CNB are recommended to undergo surgical excisional biopsy [69]. When atypia is associated with a radial scar, the risk of associated cancer is significantly increased and can be as high as 28 % [55]. This increased risk of cancer, however, does not apply to incidentally identified microscopic foci of radial scars or papillomas that are encountered in otherwise benign histology [70]. In such cases routine imaging follow-up is advised. Becker and others reported no risk of missing an associated cancer using an 11-g vacuum assisted but had a clearly unacceptable number of samples of 32 per lesion [71]. When a 14-g needle was used, the upgrade to cancer was reported in 8 % of lesions [71].
Papilloma
Following a diagnosis of a papilloma on a core needle biopsy, an upgrade to cancer has been reported to vary from 9 to 19% [72–75]. In one series all cases of cancers were identified either as interval enlargement on follow-up or development of symptoms; no cancers were seen in asymptomatic patients or in those with stable findings at follow-up [72]. In some of these studies, cancers were found near a papilloma with a study reporting subsequent of a cancer that was even 3 cm away from the papilloma raising true validity of association with a cancer [75]. One large series of 120 cases showed no cancer at excisional biopsy or on follow-up [73]. Size of the lesion has also been suggested as helpful factor in assessing risk of associated malignancy. Lesions that are 1.4 cm or greater have been shown to have a higher risk of cancer, whereas benign papillomas had a mean size of 0.9 cm (Fig. 13.7a–e). Lesions in the periphery of the breast also had a higher risk of malignancy [76, 77].
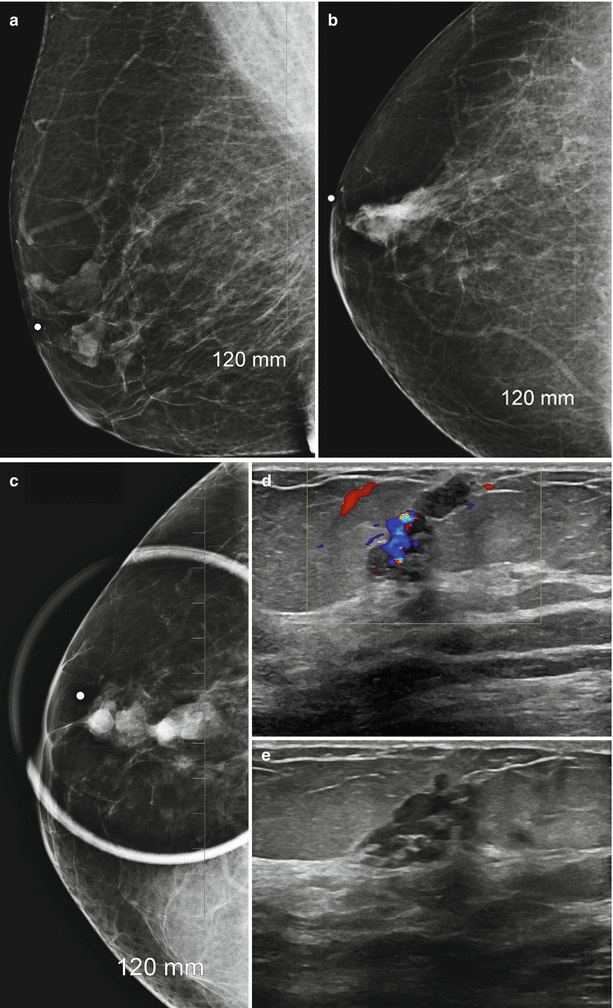

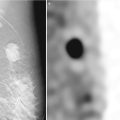
Fig. 13.7
A 67-year-old woman with a history of spontaneous bloody right nipple discharge with histologically proven papilloma associated with DCIS. (a) Mediolateral oblique view shows a retroareolar irregular tubular density. (b) Craniocaudal view shows a retroareolar irregular tubular density. (c) Spot compression craniocaudal view shows a high-density retroareolar irregular tubular density. (d) Ultrasound demonstrates an irregular intraductal mass with prominent internal vascularity. (e) Ultrasound demonstrates an irregular intraductal mass with a branching pattern
Lobular Neoplasia
Atypical lobular neoplasia and lobular carcinoma in situ carry an elevated risk for association with malignancy with LCIS having a stronger association. Atypical lobular hyperplasia is when less than 50 % of the acinar units in a lobule are distended with lobular neoplastic cells. Distension requires at least eight cells within an individual acinar unit. Reported incidence of an upgrade to malignancy in lobular neoplasia varies from as low as 1 % [78] to as high as 23 % [79]. LCIS can present as masses or calcifications [66]. One large series of 278 cases reported an upgrade to malignancy in 25 % of cases of LCIS and 22 % of ALH [78]. The overestimation of the risk of malignancy missed on a core needle biopsy in some of these series happens since cases with imaging pathological discordance or those pleomorphic or nonclassic forms of LCIS are included in the study group. In a study of LCIS upgraded to malignancy, with 9 cases of LCIS, six were in imaging pathological discordance and three included pleomorphic variant of LCIS for which surgical treatment similar to DCIS is routinely recommended [78]. Based on these findings there may not be a real justification in recommending excisional biopsy for classic forms of lobular neoplasia where there is no imaging pathological concordance. Instead imaging and clinical follow-up alone may be appropriate [78].
Flat Epithelial Atypia
Flat epithelial atypia or columnar cell change with atypia refers to presence of cystically dilated ducts that are lined by one to several layers of monomorphic but enlarged round to oval cells with low-grade cytologic atypia. Unlike that seen in ADH or DCIS, micropapillary or cribriform growth patterns are not observed in flat epithelial atypia [66]. Reported rate of DCIS and invasive cancer in flat epithelial atypia on excisional biopsy is 6–17 % [80, 81]. Mammographic microcalcifications are the most common underlying finding reported in 69 % of cases in one series followed by ultrasound finding of a hypoechoic mass in 25 % [80]. The mean size of the abnormality was 8 mm. A systematic review of 24 studies determined that the underestimation risk of DCIS in columnar cell change without atypia was 1.5 %, with atypia was 9 %, and with atypical hyperplasia was 20 %. Based on these findings it has been recommended that all cases of CCC with atypia or atypical hyperplasia undergo open surgical biopsy and lesions that demonstrate CCC without atypia can be followed [82].
Imaging Pathological Discordance
Ultrasound- and stereotactic-guided minimally invasive procedure to biopsy abnormalities in the breast has been established alternative to open surgical biopsy [83, 84]. Up to 96 % of cancers are diagnosed at initial US-guided biopsy [83]. Reasons for failed biopsy include sampling error, failure to recognize imaging pathological discordance, and lack of follow-up after benign biopsy results [83]. A large series of 1,352 cases reported a false-negative rate of 1.6 % for lesions undergoing US-guided core biopsy using a 14-g needle [84]. Imaging pathological correlation can have five different outcomes [85]. These are concordant malignancy, discordant benignity, discordant malignancy, discordant benignity, and high-risk lesions:
In concordant malignancy a lesion that was suspicious on imaging is malignant. Prompt communication to the referring clinician and the breast imager performing the biopsy or the referring clinician should contact the patient and arrange a referral to an oncologist or a breast surgeon.
In case of discordant malignancy, a lesion with benign imaging features results in a malignant histology; this should lead to management protocol as in the earlier scenario with the added step of careful review of the imaging findings as a second look to seek morphological features that may have suggested malignancy such as areas of ill-defined borders that were initially missed or associated features that are suspicious and may have been overlooked.
Concordant benignity is when a lesion considered benign is proven benign histologically. Verbal and or written communication with the referring clinician is adequate with a mechanism in place to confirm receipt of the results and proper notification of the patient with recommendation for follow-up.
Discordant benignity is when lesions are categorized as BI-RADSTM 5 or otherwise considered as suspicious for malignancy but result in nonspecific diagnosis such as benign breast tissue or fibrocystic change. There are few benign lesions that may appear probably malignant but are benign such as the desmoid tumor, fat necrosis, diabetic mastopathy, stromal fibrosis, or radial scar. However, it is safe practice to recommend excisional biopsy on all discordant benignity.
The rate of malignancy in discordant cases has been reported to be between 6.8 and 17.6 % [86–88]. The rate of malignancy in the concordant group has been reported to be low and around 0.4 % [88]. In the discordant group the upgrade was statistically significant for larger masses, in symptomatic cases, and those with a higher BI-RADSTM assessment [86–88]. In the concordant upgrade group, lesion size and symptoms were significant but not the BI-RADSTM assessment [88].
Special Histological Types of Breast Cancer
Heterogeneity of breast cancer is well recognized; about 25 % of invasive breast cancers are of special histological types [89]. Invasive ductal cancer of no special type or not otherwise specified constitutes 60–75 % of all breast cancers and is diagnosed by exclusion which is one that does not fit any special histological type [90]. At least 17 distinct entities are recognized [91]. There are also several interesting phenotypic-genotypic correlations described within this group of special tumor types; a detailed discussion on this is beyond the scope of this chapter but is described by Weigelt et al. [89]. The imaging features of the following special types are discussed here: secretory carcinoma, papillary carcinoma, mucinous carcinoma, medullary carcinoma, and tubular carcinoma. Invasive lobular cancer is discussed in the chapter on mammographic signs of malignancy (Chap. 5).
Secretory Carcinoma
Secretory carcinoma was formerly known as juvenile breast cancer and was renamed since it can occur in a wide range of age group (11–86 years). This very rare form of cancer presents at an early stage and has an indolent course. Only 120 cases have been reported in the literature [92]. The diagnosis of secretory carcinoma is made by the characteristic microscopic appearance of the tumor, with cells containing vacuolated cytoplasm and abundant intra- and extracellular secretory material. Imaging features in small series have been described and is nonspecific; lesions may be palpable or screen detected. Most commonly ultrasound demonstrates circumscribed round or oval mass either as a single nodule or multiple nodules [93].
Tubular Carcinoma
Tubular carcinomas are frequently diagnosed when small, frequently diagnosed in a younger population and have a favorable prognosis with a lower incidence of axillary metastasis. These types of cancers are rare and account for less than 2 % of breast cancers. Tubular carcinoma of the breast is a specific type of Infiltrating carcinoma that is characterized histologically by the presence of 75 % tubular structures that are lined by well-differentiated epithelial cells. The pure type is one where there are more than 90 % tubular elements and a mixed type is where there are 50–90 % tubular elements. Imaging appearances of tubular carcinoma of the breast has been reported [94–99]. Tubular carcinomas frequently appear as irregular masses with spiculated margins on mammography and are commonly visible on ultrasound as hypoechoic masses with ill-defined borders and posterior acoustic shadowing [94] (Fig. 13.8a–g). Majority are palpable [59–85 %] and appear as masses [72 %] [95, 96]. The pure type has a better prognosis and is more likely to be mammographically occult and tends to be oval shaped, whereas the mixed type is more likely to be irregular in shape and have posterior acoustic shadowing on ultrasound [97].
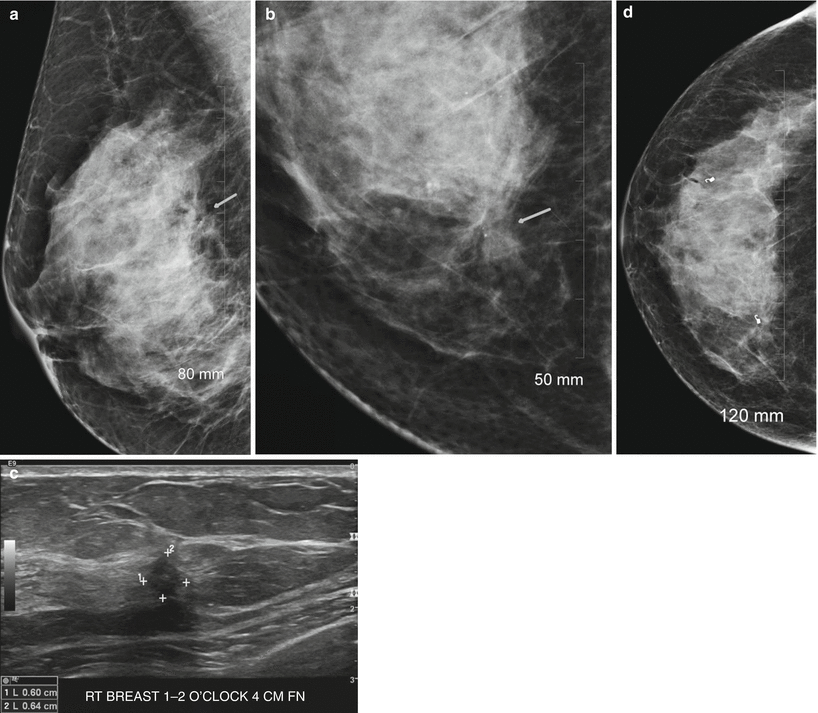
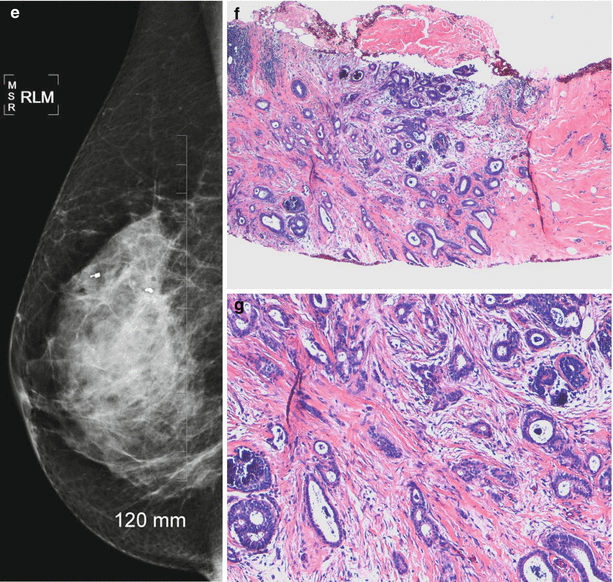


Fig. 13.8
A 46-year-old woman with histologically proven tubular cancer in the right breast. (a) Mediolateral oblique view demonstrates a subtle spiculated mass and distortion at the 1–2 o’clock position. (b) Craniocaudal view demonstrates a subtle spiculated mass and distortion at the 1–2 o’clock position associated with calcifications. (c) Ultrasound demonstrates an irregular small mass. (d) Postbiopsy craniocaudal view with a tissue marker in place. The second tissue marker corresponds to a second cancer that was histologically proven to be an invasive lobular cancer. (e) Postbiopsy mediolateral view with a tissue marker in place. The second tissue marker corresponds to a second cancer that was histologically proven to be an invasive lobular cancer. (f, g) Histology demonstrates well-defined glands with round, oval, or angulated contours, open lumina, absence of myoepithelial cell layer, and absence of necrosis or mitoses. Arrow indicates the mass described
Mucinous Carcinoma
Pure mucinous breast carcinoma is rare, occurs in older women, and is known to have a better short-term prognosis compared with infiltrating ductal cancer. It rarely presents with nodal disease and when it does it is the single significant predictor of poor prognosis. It represents about 1–4 % of breast cancer cases [100]. A large retrospective series of over 11,000 cases showed a 5-year survival rate of 94 %. The favorable outcome is maintained after 20 years; 10-, 15-, and 20-year survival rates were 89, 85, and 81 % compared to 72, 66, and 62 % for IDC [100]. Histologically there are two types, the pure type and the mixed type. Imaging features of mucinous carcinoma of the breast have been reported [101–103]. Not all mucinous carcinomas are mammographically visible; in one series 21 % were not seen on a mammogram [101]. The most common mammographic appearance is that of a circumscribed or microlobulated mass particularly in the pure mucinous type; in the mixed type the margins are ill defined or spiculated [101, 102]. At sonography the pure type of mucinous cancer is isoechoic, with posterior acoustic enhancement in a majority of cases, 63 % in one series [103] (Fig. 13.9a–e). Mixed cystic and solid components and microlobulated borders have also been described [101]. Internal echotexture tends to be homogenous in the pure type of mucinous carcinoma and hypoechogenic in the mixed type [101, 102].