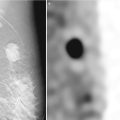
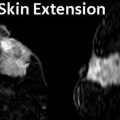
Fig. 11.1
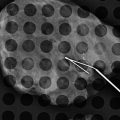
A 16-year-old with a palpable mass histologically proven to be a fibroadenoma at core-needle biopsy. Ultrasound shows an ovoid circumscribed mass with homogenous internal echotexture
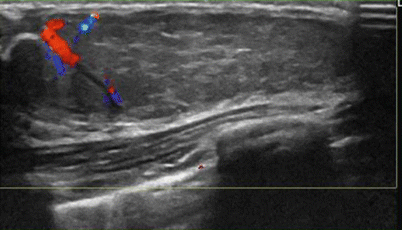
Fig. 11.2
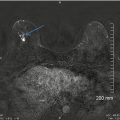
A 15-year-old with a large palpable mass histologically proven to be a juvenile fibroadenoma. Ultrasound shows a large circumscribed ovoid mass with a peripheral cleft and vascularity
Pseudoangiomatous Stromal Hyperplasia (PASH)
Pseudoangiomatous stromal hyperplasia (PASH) is an entity that is generally seen in older women. It is histologically characterized by hormonally stimulated proliferations of myofibroblasts; rarely they may be seen in late adolescence and uncommonly grow rapidly. Clinically and on imaging, these lesions can have features similar to fibroadenomas. The mean size of these tumors is 4.2 cm with a range between 1 and 11 cm [9]. The name is derived from the characteristic histological feature of anastomosing slit-like channels lined by flat myofibroblastic cells that resemble endothelial cells and is surrounded by dense stroma [1]. When red blood cells are seen within these slit-like spaces on a core biopsy, it can be confused with an angiosarcoma [10]. On sonography, these masses appear as an oval circumscribed mass with margins that may be less well defined than a typical fibroadenoma; there may be a posterior acoustic enhancement associated with this mass. Conservative management after a diagnosis of PASH is recommended with excision reserved for symptomatic or enlarging masses.
Juvenile Papillomatosis
Juvenile papillomatosis is a localized benign proliferative disorder that is uncommonly seen in teens with a mean age of diagnosis at 19 years [11]. Histologically, there are multiple cysts and dilated ducts in a dense stroma, an appearance that has been characterized as “Swiss cheese disease.” Mammography may show an area of focal asymmetry or microcalcifications [12]. At sonography, an ill-defined mass with multiple cystic areas of varying sizes is seen at the periphery of the lesion [12]. Juvenile papillomatosis is a marker for familial breast cancer. About 5–15 % of patients have concurrent breast cancer and 33–58 % of cases have a positive family history of breast cancer [13, 14].
Intraductal Papilloma
Intraductal papillomas are rare in children and when seen appear as solitary circumscribed masses in a dilated duct and may be outlined by secretions within a duct. These tend to occur in the larger subareolar ducts. In 25 % of cases, papillomas may be bilateral [2]. Nipple discharge may be a presenting symptom. Histologically, these papillomas resemble juvenile papillomatosis.
Granular Cell Tumor
Granular cell tumor is a rare benign tumor; about 5–6 % of these occur in the breast and most commonly in premenopausal African American women [15]. These account for 1 % of breast tumors in children and originate from perineural cells. Granular cell tumors appear as 1–2 cm superficially located firm masses and may be associated with skin fixation or retraction. At mammography, they may appear as a spiculated mass or as a circumscribed mass. On ultrasound, the spiculated mass may demonstrate posterior acoustic shadowing and appear malignant. These are treated with wide excision when a histological diagnosis is achieved with percutaneous biopsy.
Malignant Tumors
Phyllodes Tumor
Phyllodes tumor is the most common primary breast malignancy in the adolescents. It is a stromal tumor arising from the lobular connective tissue [7]. A higher incidence has been reported in those with an Asian heritage. These tumors present as rapidly enlarging breast lumps. The benign types of phyllodes are more common in this age group. Phyllodes tumors occur predominantly in older women; however, about 5 % of these tumors are seen in girls younger than 20 years. Clinically, pathologically, and at imaging, these tumors may resemble a juvenile type of fibroadenoma [1]. Most tumors are larger than 6 cm with an average range of 8–10 cm [2]. A size less than 4 cm has a favorable prognosis as does presence of pushing rather than infiltrative borders, lack of necrosis, and lesser than three mitosis per high power field [1].
At sonography, these masses are circumscribed but tend to have a heterogeneous internal echotexture with clefts and cystic spaces compared to the more homogenous areas within a fibroadenoma (Fig. 11.3a, b). Mammography demonstrates a hyperdense circumscribed mass without calcifications; malignant types may exhibit pleomorphic microcalcifications. Recurrence is a feature noticed in both benign and malignant types; the former recurs about 10–25 % of the time and the recurrence rate in malignant phyllodes is greater than 40 % even following a wide excision [4]. About 5–24 % of phyllodes in those less than 20 years of age are malignant. Metastasis is uncommon but when present is hematogenous and to the lungs. Malignant types are histologically associated with sarcomatous elements, infiltrative margins, necrosis, cellular atypia, and increased stromal cellularity.
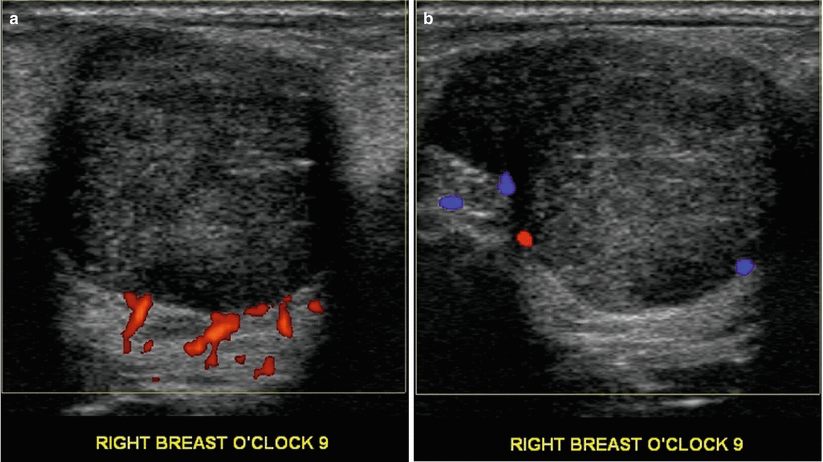

Fig. 11.3
(a, b) An 18-year-old with a rapidly enlarging palpable mass histologically proven to be a benign phyllodes tumor at excisional biopsy. Ultrasound demonstrates a round solid mass with ill-defined margins
Carcinoma
Breast carcinoma in girls under the age of 20 years is exceedingly rare, and based on the National Cancer Institute’s Surveillance Epidemiology and End Results data from 2006 to 2010, 0.0 % was diagnosed [16]. Less than 1 % of breast lesions in children is caused by breast cancer [17]. The most common type is the secretory carcinoma that presents as a circumscribed mass less than 3 cm and with a pseudo capsule [1, 11]. Prognosis is favorable. Breast cancer in these girls may be related to inherited BRCA1 and BRCA2 mutations [11]. Sonographic appearance is that of a malignant mass with irregular margins, taller than wide and with posterior acoustic shadowing.
Metastasis
The most prevalent malignant tumors in the breast in children and adolescents are metastasis. The most common tumors metastasizing to the breast are rhabdomyosarcoma, neuroblastoma, and hematolymphoid malignancies [1, 18]. Breast metastasis has been reported in 6 % of patients with rhabdomyosarcoma [18]. Metastasis are frequently bilateral and multiple although a solitary mass may also be seen. Clinically, masses may exhibit rapid growth and be painful. Sonographic appearance is variable and may exhibit a spectrum of appearance leading to an indeterminate morphology prompting a tissue diagnosis.
Pregnancy and Lactation: Benign Abnormalities of the Breast
The physiologic changes occurring in pregnancy are induced by high circulating levels of estrogen and progesterone, and these lead to increase in breast size, its firmness, and increased nodularity. These changes continue for 3 months after cessation of lactation. Physical examination is difficult as a result of these changes in the breast and it is advised that a baseline clinical breast exam is performed during the first visit to the obstetrician [19]. Mammographic increase in density of the breast also limits its value during pregnancy and lactation. Pumping of the breast prior to a mammogram is also helpful. Ultrasound demonstrates diffuse increase in the echogenicity of normal breast tissue during pregnancy and lactation. During lactation, one sees distended ducts as hypoechoic tubular structures. Increased vascularity is also an expected feature [20].
The most common symptoms prompting imaging in pregnancy and lactation are a palpable lump, mastitis, and a bloody nipple discharge. Ultrasound is the initial imaging modality of choice and often the only modality that is utilized. Mammography is generally not performed in pregnancy although the radiation risk to the developing fetus is insignificant [21]. MRI of the breast is not an option during pregnancy since the safety of intravenous gadolinium has not been established in pregnancy. During lactation, MRI may be used if needed with instructions to stop breastfeeding for 24 h after [21].
Spontaneous Nipple Discharge in Pregnancy
Nipple discharge in pregnancy is uncommon; cytological examination of spontaneous nipple discharge is performed. If no pathology is seen and physical and ultrasound examination is normal, clinical follow-up is advised. If pathologic results are seen on cytology, galactography may be indicated to exclude an intraductal lesion such as a papilloma. Nipple discharge is an uncommon manifestation of pregnancy-associated breast cancer [21].
Fibroadenoma
Fibroadenomas being hormone-sensitive tumors may manifest varied appearances in pregnancy and lactation due to superimposition of hormone-induced and or lactational changes. Previously unsuspected fibroadenomas may be discovered in pregnancy due to enlargement and becoming palpable or symptomatic. Palpable fibroadenomas generally require tissue diagnosis that is best achieved with percutaneous core biopsy. Nonpalpable solid masses without suspicious morphology may be followed. The appearance of a fibroadenoma on ultrasound in pregnancy and lactation is varied and the following descriptions have been used [21]. Gravid fibroadenomas may demonstrate large cysts, dilated ducts, or increased vascularity (Fig. 11.4). Fibroadenoma with infarction may present as a painful tender mass. Intravascular thrombi have been identified on occasion in these fibroadenomas; this occurs usually in the third trimester. A more lobulated contour, heterogeneous architecture and posterior acoustic shadowing may be seen in such tumors as a result of infarction. Fibroadenoma with lactational changes and secretory hyperplasia demonstrates dilated ducts within, hyperechogenicity, and cystic changes. Aspiration may sometimes reveal milk as in a galactocele; distinction from a lactating adenoma may be difficult as well although this is not a management issue. Lactational adenoma histologically lacks the myoepithelial proliferation that is characteristic of fibroadenoma.
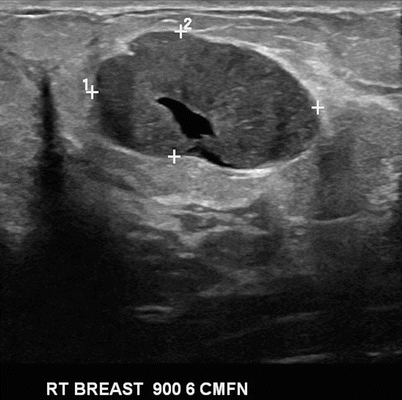

Fig. 11.4
A 28-year-old pregnant woman with a palpable lump. Ultrasound demonstrated a circumscribed ovoid solid mass with a central tubular cystic structure consistent with gravidic fibroadenoma
Galactocele
Galactoceles are the most common benign breast lesions in lactating women but are more frequently seen after cessation of lactation due to stagnation and retention of milk in the breast [21]. These are cysts that are lined with flat or cuboidal epithelium containing fluid that resembles milk that contains a variable amount of protein, fat, and lactose. The underlying etiopathogenesis is ductal dilatation, surrounding fibrous wall, and varying degrees of inflammation. Aspiration is diagnostic and therapeutic. The imaging appearance is variable depending on the contents:
Pseudolipoma is when the entire content is fat in which case mammographically a lucent circumscribed mass is encountered that sonographically appears uniformly hyperechoic or hypoechoic (Figs. 11.5a–c and 11.6a–c). Cystic mass with fat fluid level is when there is a mixture of fat that rises on top of water contents leading to a fat fluid level seen on mediolateral mammograms and on ultrasound. This sign is characteristic of a galactocele but may occasionally be seen in fat necrosis (Fig. 11.7a–f).
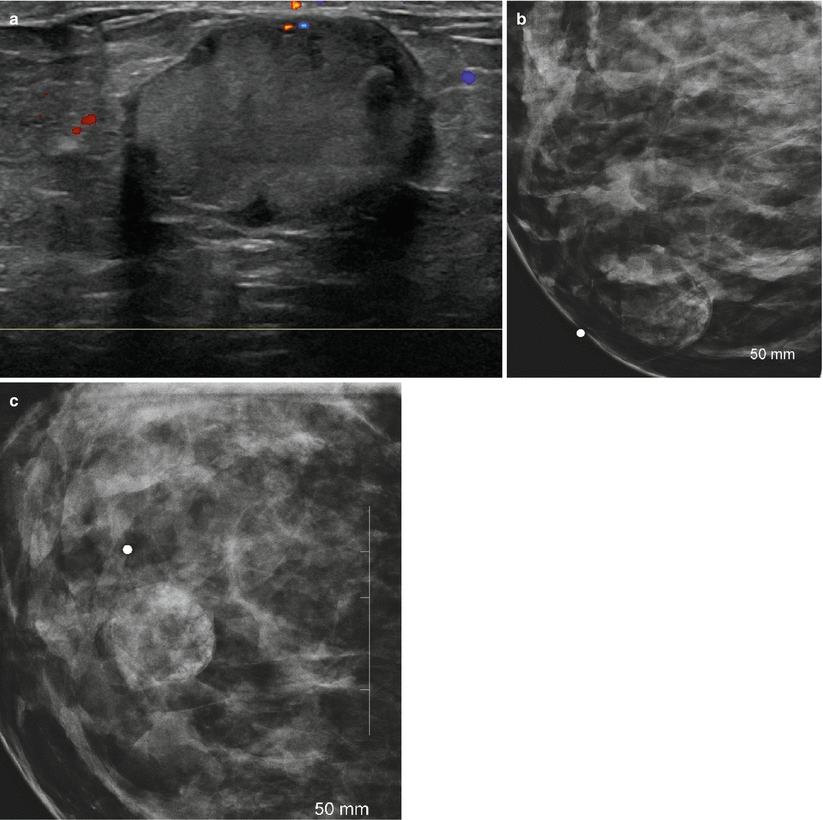
Fig. 11.5
(a–c) A 31-year-old lactating woman with a palpable lump histologically proven to be a galactocele. (a) Ultrasound demonstrated a hyperechoic circumscribed solid mass. (b) Spot compression view in the mediolateral projection shows a mixed fat and soft tissue mass. (c) Spot compression view in the craniocaudal projection shows a mixed fat and soft tissue mass
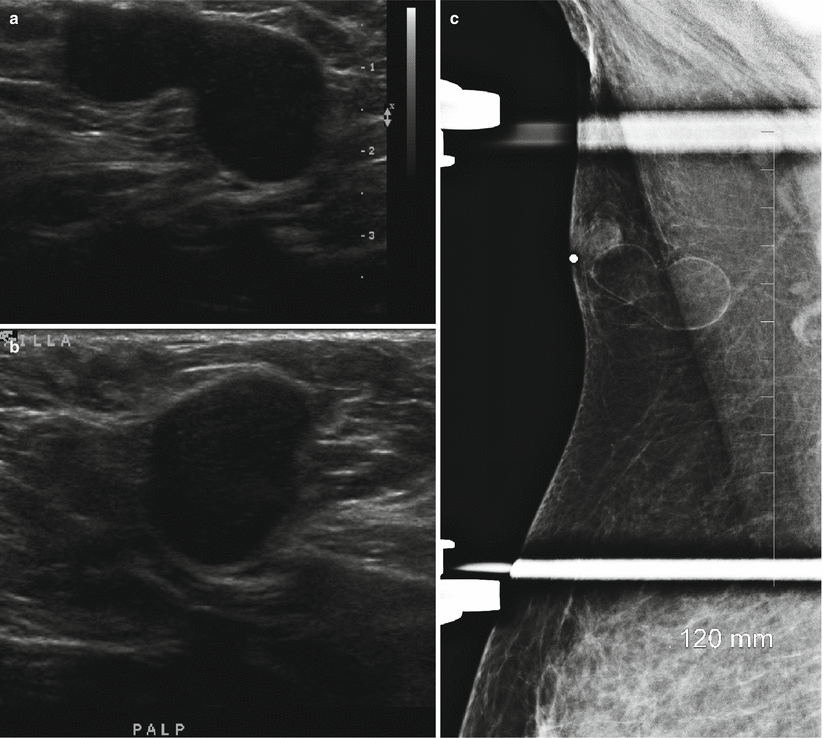
Fig. 11.6
(a–c) A 31-year-old with a palpable lump in the right axilla adjacent to an accessory nipple consistent with a galactocele. (a) Ultrasound demonstrates a circumscribed solid hypoechoic mass with a shape simulating an enlarged abnormal axillary lymph node. (b) Ultrasound demonstrates a circumscribed solid hypoechoic mass with a shape simulating an enlarged abnormal axillary lymph node. (c) Spot compression mammographic view shows the ultrasound solid-appearing mass to be radiolucent mass consistent with a benign finding. The round density contiguous with this lesion corresponded to the accessory nipple
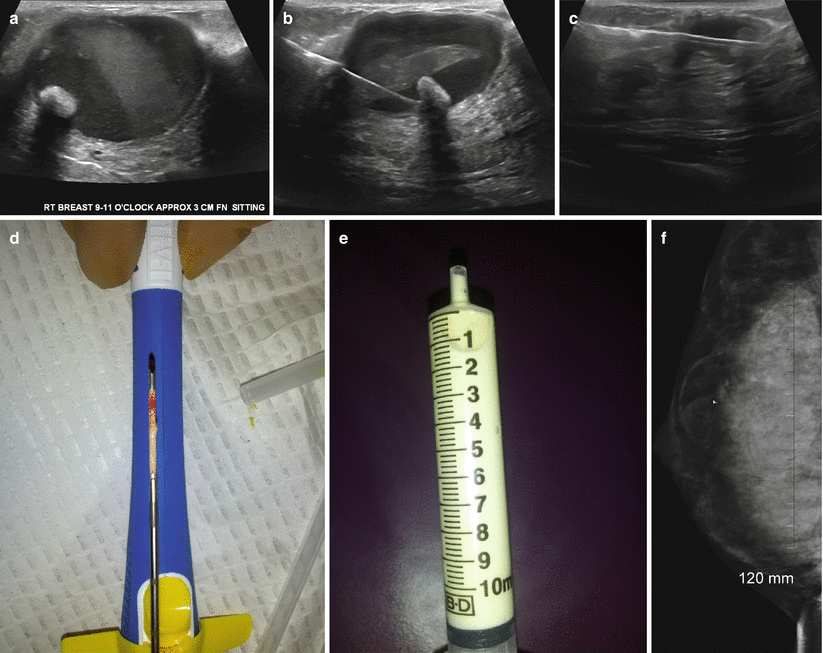
Fig. 11.7
(a–f) A 28-year-old lactating woman with a painful palpable lump histologically proven to be a galactocele. (a) Ultrasound demonstrated a complex cystic mass with echogenic contents and a mural nodule showing posterior acoustic shadowing. (b) US-guided aspiration with needle in the cystic mass and within the mural nodule. (c) Post core-needle biopsy, the cavity is partially collapsed. (d) Core specimen from the mural nodule and cyst wall. (e) Milky aspirate from the galactocele in a syringe. (f) Post-biopsy mediolateral oblique view demonstrates a fat density mass with the post-biopsy clip and a fat fluid level
Pseudohamartoma is an appearance more often seen in chronic galactocele where a mixed fat and soft tissue density mass is seen resembling a hamartoma. Galactoceles can get infected and painful; aspiration in such cases may reveal purulent material with positive culture.
Galactocele can have a complex cystic mass with thick internal septations. Aspiration causes the lesion to partially collapse and reveals milk-like aspirate (Figs. 11.7a–f and 11.8a, b).
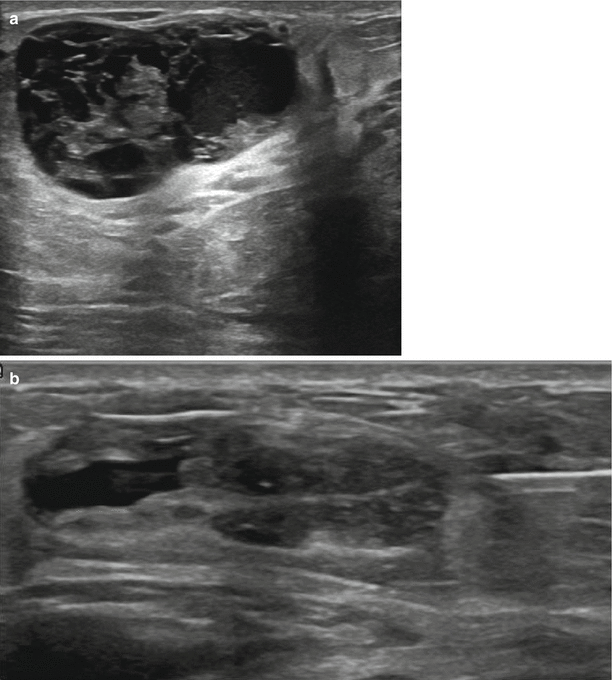

Fig. 11.8
(a, b) A 32-year-old woman with a palpable tender lump during lactation revealed a complex galactocele at biopsy. (a) Ultrasound demonstrates a complex cystic mass with thick internal septations. (b) Post ultrasound-guided core biopsy, the lesion is partially collapsed
Lactating Adenoma
Lactating adenomas are benign tumors of the breast typically seen in the third trimester and during lactation [20]. These masses resemble fibroadenoma clinically and on imaging appear as circumscribed mobile oval-shaped masses. When infracted, they appear as firm tender masses [22]. Histologically unlike a fibroadenoma, these have very little stromal elements and consist predominantly of epithelial elements. These elements consist of mature tubules containing actively secreting cells filling up the acini with secretions [22]. Lactating adenomas uniquely tend to regress after cessation of breastfeeding [23]. At sonography, posterior acoustic enhancement and increased lesion compressibility is characteristic likely due to large amount of secretions in the acini (Figs. 11.9a, b, 11.10a–c, and 11.11a–c) [22]. Infarction leads to appearance of irregular margins and posterior acoustic shadowing.
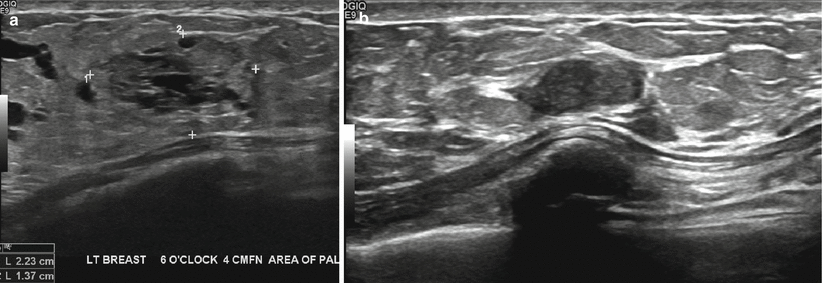
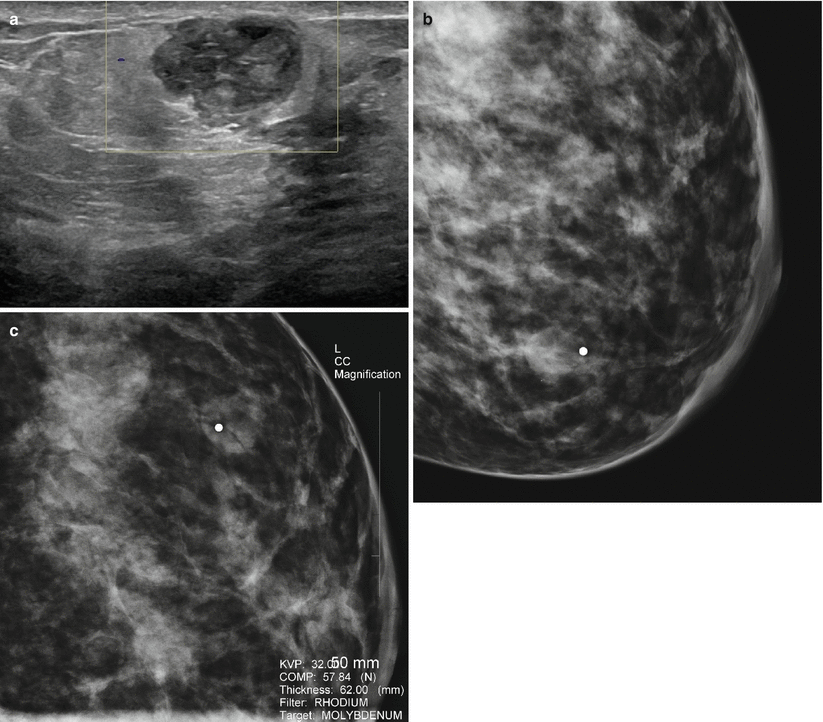
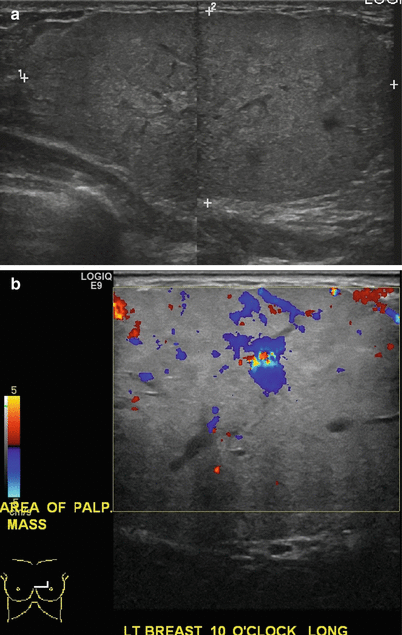

Fig. 11.9
(a, b) A 34-year-old woman with a palpable lump during lactation revealed a lactating adenoma. (a) Ultrasound demonstrates a solid mass with multiple tubular cystic structures within representing dilated ducts. (b) Follow-up ultrasound at 12 months reveals the mass being smaller and without the lactational changes of dilated ducts

Fig. 11.10
(a–c) A 34-year-old with a palpable lump histologically proven to be an infracted lactating adenoma. (a) Ultrasound demonstrated a solid indeterminate mass. (b) Mediolateral oblique mammogram shows a round dense mass with obscured borders. (c) Craniocaudal mammogram shows a round dense mass with obscured borders

Fig. 11.11
(a, b) A 36-year-old with a palpable lump in the left breast with histologically proven infracted lactating adenoma. Ultrasound demonstrates a superficially located solid mass with heterogeneous echotexture and circumscribed lobulated borders. Appearance was consistent with an indeterminate mass with a recommendation for ultrasound-guided core biopsy
Juvenile Papillomatosis
There is an increased frequency of benign proliferative disease during pregnancy and lactation [21]. Juvenile papillomatosis is generally seen in young women. An association with pregnancy has been proposed based on finding 5 cases of this entity in a series of 18 pregnant patients [21]. On ultrasound, juvenile papillomatosis appears as an ill-defined mass that is composed of multiple cysts surrounded by fibrous septa and well demarcated histologically from surrounding tissue. The cystic and ductal hyperplasia is associated with papillary hyperplasia lining the cystic spaces [24]. Definitive treatment is by surgical excision with negative margins required to avoid local recurrence [21]. In young women, juvenile papillomatosis is a risk factor for breast cancer with a reported association with breast cancer in 15 % of cases and a reported incidence of breast cancers in up to 50 % of female relatives [21, 24].
Granular Cell Tumor
This is a rare benign tumor seen in young women. About 5–6 % are seen in the breast and more commonly seen in African American women. They arise from perineural cells. Clinically, these present as superficial firm masses and there may be associated skin changes [1]. Histologically, they tend to form an infiltrative growth and simulate an infiltrative carcinoma, clinically and on imaging. At sonography, these appear as 1–2 cm irregular masses with posterior acoustic shadowing and tend to exhibit characteristics of a malignant mass (Fig. 11.12a, b). At mammography, these may appear as spiculated masses and simulate invasive ductal cancers; these can also appear as well-circumscribed masses. Despite the malignant appearance on imaging, these tumors are benign and preoperative diagnosis is important to treat appropriately with wide excision [1].
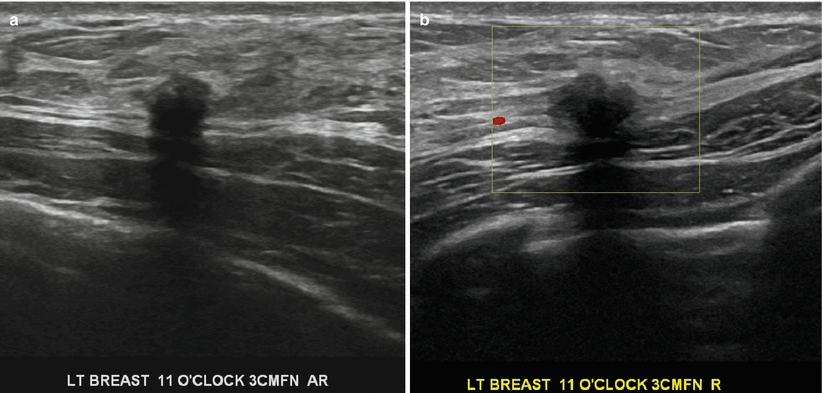

Fig. 11.12




(a, b) A 29-year-old woman postpartum with a hard palpable lump histologically proven to be a granular cell tumor. Ultrasound demonstrates a small irregular hypoechogenic mass with posterior acoustic shadowing that was prebiopsy categorized as highly suggestive of malignancy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree