Multiple myeloma is the most common bone malignancy. Imaging plays an important role in identifying the extent of the disease, disease process, guiding biopsies, and diagnosing associated spinal and intracranial complications. Multiple myeloma and related plasma cell proliferative disorders have a diverse set of clinicopathologic findings and on neuroimaging present unique and diverse findings from the disease and from complications of the disease and treatment, which are valuable for clinicians and radiologists.
Key points
- •
Diagnosis of multiple myeloma is based on a combination of clinical findings, laboratory studies, bone marrow biopsy, and imaging findings.
- •
Imaging plays an important role in identifying the extent of the disease, disease process, guiding biopsies, and diagnosing associated spinal and intracranial complications. It also plays an important role in the staging, evaluating response to therapy, and monitoring for recurrence.
- •
Multiple myeloma and related plasma cell proliferative disorders (PCPDs) have a diverse set of clinicopathologic findings and with those present unique and diverse findings on neuroimaging, not only from the disease itself but from complications of the disease and treatment-related complications. Familiarity with these findings is valuable for clinicians and radiologists alike.
- •
This article describes the imaging findings associated with common neurologic complications seen with multiple myeloma and related PCPDs.
Introduction
Multiple myeloma is a malignant neoplasm of plasma cells that produces monoclonal immunoglobulins. Each year in the United States, approximately 20,000 new cases are diagnosed with approximately 11,000 deaths. Most patients are older than 60 years with a median age at diagnosis of 66 years old.
Multiple myeloma is a cytogenetically and molecularly diverse neoplasm leading to a wide range of clinical disease. Multiple myeloma is believed to be a progression of a premalignant stage called monoclonal gammopathy of undetermined significance (MGUS). Patients present at varying stages from the premalignant MGUS, to an intermediate asymptomatic stage termed smoldering myeloma, to the symptomatic stage termed multiple myeloma. Patients may also present at a stage before multiple myeloma and progress all the way to multiple myeloma at varying rates depending on several cytogenetic and molecular factors.
MGUS is predominantly seen in patients older than 50 years with a prevalence of approximately 3% in the population 50 years and older, 5% in the population 70 years and older, and 7.5% in the population 85 years and older.
The purpose of this review article is to describe the imaging findings associated with common neurologic complications seen with multiple myeloma and related plasma cell proliferative disorders (PCPDs). The diagnosis, classification of multiple myeloma and PCPDs, staging and risk stratification, and treatment are briefly described as they pertain to neurologic complications. A brief overview of the imaging of multiple myeloma and then specific imaging findings associated with common neurologic complications seen with multiple myeloma are described.
Introduction
Multiple myeloma is a malignant neoplasm of plasma cells that produces monoclonal immunoglobulins. Each year in the United States, approximately 20,000 new cases are diagnosed with approximately 11,000 deaths. Most patients are older than 60 years with a median age at diagnosis of 66 years old.
Multiple myeloma is a cytogenetically and molecularly diverse neoplasm leading to a wide range of clinical disease. Multiple myeloma is believed to be a progression of a premalignant stage called monoclonal gammopathy of undetermined significance (MGUS). Patients present at varying stages from the premalignant MGUS, to an intermediate asymptomatic stage termed smoldering myeloma, to the symptomatic stage termed multiple myeloma. Patients may also present at a stage before multiple myeloma and progress all the way to multiple myeloma at varying rates depending on several cytogenetic and molecular factors.
MGUS is predominantly seen in patients older than 50 years with a prevalence of approximately 3% in the population 50 years and older, 5% in the population 70 years and older, and 7.5% in the population 85 years and older.
The purpose of this review article is to describe the imaging findings associated with common neurologic complications seen with multiple myeloma and related plasma cell proliferative disorders (PCPDs). The diagnosis, classification of multiple myeloma and PCPDs, staging and risk stratification, and treatment are briefly described as they pertain to neurologic complications. A brief overview of the imaging of multiple myeloma and then specific imaging findings associated with common neurologic complications seen with multiple myeloma are described.
Classification of myeloma, monoclonal gammopathy of undetermined significance, and related plasma-cell proliferative disorders
The International Myeloma Working Group (IMWG) has set diagnostic criteria for multiple myeloma and MGUS, the most recent revised criteria of 2014 is described in ( Box 1 ). The IMWG divides the premalignant MGUS and related PCPDs into 6 classes based on several clinicopathologic criteria; non-immunoglobulin (Ig)M MGUS, IgM MGUS, light chain MGUS, solitary plasmacytoma, POEMS syndrome, and systemic amyloid light chain (AL) amyloidosis. As previously described, each of these can then progress onto an intermediate stage before becoming multiple myeloma. Also, as described, patients can present at any stage from MGUS to myeloma.
Non-IgM MGUS
IgM MGUS
Light chain MGUS
Solitary plasmacytoma
POEMS syndrome
Systemic AL amyloidosis
Immunoglobulin M, non-immunoglobulin M, and light chain monoclonal gammopathy of undetermined significance
The first 3 divisions are based on the monoclonal or M-protein, which is secreted by the clonal plasma cells. There are the non-IgM MGUS, IgM MGUS, and light chain MGUS. The IgM MGUS is usually compromised of IgG, IgA, and much less frequently IgD and IgE monoclonal gammopathies. The PCPD is classified as MGUS when the serum monoclonal protein is present but less than 30 g per liter and clonal bone marrow cells are less than 10%. In addition, there must be no end-organ damage from the monoclonal gammopathy such as hypercalcemia, renal insufficiency, anemia, and bone lesions (the so called CRAB features) or amyloidoisis. Smoldering myeloma will have a non-IgM serum monoclonal protein more than 30 g per liter and/or clonal bone marrow cells more than 10% but no end-organ damage. Multiple myeloma is diagnosed with evidence of end-organ damage.
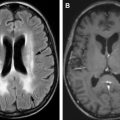
The diagnosis of IgM MGUS, smoldering IgM myeloma and multiple myeloma (with an IgM monoclonal M-protein) is similar but with an IgM serum monoclonal protein meeting criteria as previously described. In addition to smoldering IgM myeloma and multiple myeloma, there are other unique subclassifications of the IgM monoclonal gammopathy termed Waldenström macroglobulinemia (WM) and its associated intermediate form, smoldering WM. The diagnosis of smoldering WM and WM requires both serum and bone marrow minimums with distinct cytogenetic of clonal cells, which distinguish it from multiple myeloma ( Fig. 1 ).
The diagnosis of light chain MGUS is based on an abnormal serum free light chain ratio (<0.26 with elevated lambda light chain or >1.65 with elevated kappa light chain) and no heavy chain immunoglobulin. Light chain MGUS also requires less than 10% clonal bone cells, less than 500 mg per 24 hours of urinary monoclonal protein, and lack of end-organ damage. The light chain monoclonal gammopathy can progress to an intermediate stage termed idiopathic Bence Jones proteinuria. Idiopathic Bence Jones proteinuria is diagnosed with a urinary monoclonal protein greater than 500 mg per 24 hours without end-organ damage. It is termed light chain myeloma with evidence of end-organ damage. Progression rates to multiple myeloma have been shown to be about 1% per year for IgM and non-IgM MGUS to multiple myeloma. Light chain progresses less frequently at about 0.3% per year.
Solitary plasmacytoma, POEMS syndrome, and systemic amyloid light chain amyloidosis
The last 3 divisions of premalignant disorders are based on other unique clinicopathologic criteria due to distinctness of the disease entity, including disease progression rate and/or prognosis. These include solitary plasmacytoma, POEMS syndrome, and systemic AL amyloidosis.
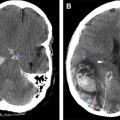
Solitary plasmacytoma is diagnosed by biopsy of solitary bone or soft tissue lesion (also termed medullary or extramedullary, respectively) demonstrating clonal plasma cells. Solitary plasmacytoma must show normal bone marrow without evidence of plasma clonal cells. There is a subcategory in which there are less than 10% clonal bone marrow cells, which is termed solitary plasmacytoma with minimal marrow involvement. This has a much a higher progression rate to multiple myeloma (60% at 3 years) compared with solitary plasmacytoma without marrow involvement (10% at 3 years). There must be no other lesions seen on imaging except for the solitary plasmacytoma ( Fig. 2 ). There must also be absence of end-organ damage.
POEMS syndrome is an acronym for polyneuropathy, organomegaly, endocrinopathy or edema, M-protein, and skin changes. The diagnosis is made by demonstrating the combination of polyneuropathy, a monoclonal PCPD (usually lambda), 1 of 3 major criteria (sclerotic bone lesion, Castleman disease, elevated serum vascular endothelial growth factor [VEGFA] levels), and 1 of 6 minor criteria (organomegaly, volume overload, endocrinopathy, skin changes, papilledema, thrombocytosis or polycythemia). Unlike the osteolytic bone lesions seen in multiple myeloma, the bone lesions seen in POEMS syndrome are typically osteoblastic (or sclerotic), which can mimic metastatic prostate cancer and other osteoblastic lesions ( Fig. 3 ).
Systemic AL (immunoglobulin light chain) amyloidosis is diagnosed by evidence of an amyloid-related systemic syndrome, such as renal, liver, heart, gastrointestinal, or peripheral nerve involvement. There must be positive amyloid staining by Congo red of biopsied tissue and the amyloid must be composed of light chain. In addition, there must be evidence of a monoclonal PCPD, as described previously, in light chain MGUS. When patients meet criteria for multiple myeloma and systemic AL amyloidosis they are considered to have both diseases.
Plasma cell leukemia is a distinct entity not typically described with MGUS and myeloma, although it will be described here for completeness. This can occur either de novo, in which it is called primary plasma cell leukemia, or it can occur as a relapsed or refractory multiple myeloma, in which it is called secondary plasma cell leukemia. Plasma cell leukemia is a rare and very aggressive entity distinguished by high levels of plasma cells circulating in the peripheral blood (>2000 per microliter).
Multiple myeloma staging and risk stratification
Multiple myeloma is divided into stage I, II, and III based on 2 classification systems: the International Staging System (ISS) and Durie-Salmon staging (DSS). The ISS uses only beta-2 microglobulin and albumin levels to distinguish between the stages. DSS uses hemoglobin levels, calcium levels, serum immunoglobulin levels, urine monoclonal protein levels, and bone lesions seen on imaging to distinguish between stages. Bone lesions were historically evaluated with skeletal surveys and divided into no lytic lesions for stage I and advanced lytic lesions for stage III. In the DSS system there are also (a) and (b) subclassifications based on serum creatinine levels. In a revised 2006 system of the DSS, termed the DSS Plus, advanced imaging (PET-computed tomography [CT] or MRI) was introduced into the staging to better identify and prognosticate patients who were previously lumped together, yet had distinctly different disease prognostics. Now stage II lesions are better differentiated with this classification as stage I having less than 4 lesions, stage II having 5 to 20 lesions, and stage III having more than 20 seen on advanced imaging.
In addition to staging, there is risk stratification because patients with the same stage, based on the 2 aforementioned staging systems, may have drastic differences in prognosis based on differences in cytogenetics and laboratory values not accounted for.
Multiple myeloma treatment
MGUS patients do not meet criteria for treatment. Asymptomatic myeloma patients with certain criteria (bone marrow plasmacytosis >60%, markedly elevated serum light chain levels, and bone marrow involvement on MRI or CT-PET) may be eligible for treatment, depending on several other patient factors.
Solitary plasmacytoma is usually treated with radiation therapy alone to the site with monitoring for progression to multiple myeloma in which the treatment would then progress (see later discussion).
Patients are split into 2 main groups: autologous stem-cell transplant (ASCT)–eligible and non-ASCT–eligible. In the 2013 Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines, “physiologic age less than 70 years old” was used as a cutoff for ASCT eligibility. Other criteria of exclusion commonly used are age greater than 77 years old, direct bilirubin greater than 2.0 mg/dL, Eastern Cooperative Oncology Group (ECOG) performance status 3 or 4, or New York Heart Association functional class III or IV.
In the revised mSMART, both ASCT eligible and ineligible patients were classified into standard, intermediate, and high-risk groups to guide therapy. High-risk eligible patients receive 4 cycles bortezomib-lenalidomide-dexamethasone (VRD) for induction therapy then go onto ASCT (especially if not in complete remission), with VRD maintenance for a minimum of 1 year. Intermediate eligible receive 4 cycles of cyclophosphamide-bortezomib-dexamethasone (CyBorD) then go onto ASCT, followed by bortezomib-based therapy for minimum of 1 year. Standard-risk patients receive 4 cycles of dexamethasone (Rd) or CyBorD, then will either go onto ASCT with consideration of lenalidomide maintenance therapy or Rd maintenance alone without ASCT. High-risk ineligible patients get VRD. Intermediate ineligible patients receive melphalan-prednisone (MP) plus weekly CyBorD or weekly bortezomib-based therapy, then go onto bortezomib maintenance. Standard-risk ineligible patients get Rd or MP plus thalidomide, then go onto observation.
These common treatment regimens used in myeloma are important in neurologic imaging because many complications of treatment affect the neurologic system in a specific way and have associated imaging findings.
Imaging of multiple myeloma
Radiography
Skeletal survey has historically been the recommended imaging for detecting myeloma bone disease. On radiography, multiple myeloma lesions typically have a punched-out osteolytic appearance. However, at least 50% of the involved trabecular bone needs to be destroyed to be detectable as a lytic lesion on radiography. In some cases, up to 75% of the trabecular bone needs to be destroyed before showing up on radiography.
Skeletal lesions can be seen in the spine, pelvis, skull, ribs, sternum, and proximal appendicular skeleton, in order of frequency. Although rare, more distal appendicular skeleton can be affected. Four types of involvement have been described on skeletal radiography: the solitary lesion (plasmacytoma), diffuse skeletal involvement (myelomatosis), the most common diffuse skeletal osteopenia, and the rare presentation of sclerosing myeloma.
Computed Tomography
CT compared with radiography is much more sensitive in detecting bone lesions. Lesions with less than 5% of trabecular bone destruction can be detected compared with radiography ( Fig. 4 ). In addition, CT can be used to evaluate for involvement and compromise of surrounding soft tissues. Body CT can also detect soft tissue extramedullary plasmacytomas. CT is commonly used in guiding biopsies of MRI-detected lesions. Although CT poses higher dose of radiation, low-dose, whole-body CT protocols have been used with similar sensitivities but with a much lower dose of radiation.
Nuclear Medicine Studies
Several nuclear medicine studies have been used to evaluate myeloma. Tc-99m bone scans have been attempted but show poor results due to the lack of osteoblastic activity, unless there has been a complication such as a pathologic fracture.
Galium-67 (Ga), thallium-201 (Tl), and fluorodeoxyglucose F-18 positron emission tomography (PET) have all been used to detect myeloma. Due to their ability to detect physiologically active disease, these studies were very beneficial not only for detecting disease but for following treatment response. However, Ga and Tl are rarely used today due to several technical difficulties that make them obsolete. PET-CT on the other hand is becoming more widespread and is supplementing MRI. PET-CT can detect active disease and may be better than MRI at monitoring response to therapy. In addition, PET-CT is excellent at evaluating extramedullary disease. Like MRI, PET-CT is included in the DSS Plus system for staging with advanced imaging.
MRI
MRI has now become the gold standard for imaging in multiple myeloma. The IMWG released a consensus statement in 2015 stating the indications of MRI in multiple myeloma and PCPDs. MRI is very sensitive and specific for detecting bone disease and soft tissue involvement or compromise in myeloma.
Detecting myeloma bone disease on MRI is based on replacement of normal bone marrow with neoplastic cells. Normal bone marrow appearance depends on the age of the patient and location of the bone marrow. The axial skeleton is much richer in red marrow than the appendicular skeleton, which is comprised mostly of fatty marrow. As humans age, even the axial skeleton is progressively replaced with fatty marrow. On MRI, this can be seen by the progressive increase in T1-weighted signal intensity in the marrow. The frequency of T1 change from hypointense to iso-hyperintense at different age groups is described by Ricci and colleagues. Most patients with myeloma are more than 50 years old and have fatty replaced marrow, which is T1 hyperintense and hypointense on fluid-sensitive sequences (T2-weighted and short-tau inversion recovery [STIR]). Therefore, a hypointense lesion on T1 and hyperintense lesion on fluid-sensitive sequences will indicate marrow replacement in this group ( Fig. 5 ). Fluid-sensitive sequences tend to be the most sensitive at detecting lesions, with STIR usually even more so than standard T2 due to fat suppression. It is important to realize this is a nonspecific sign because this can be seen in other marrow replacing diseases such as metastatic disease, lymphoma and leukemia. Also, caution must be taken when evaluating diffuse band-like T1 hypointensities, which can be a normal finding in patients more than 60 years of age.