Image-guided percutaneous biopsy is the technique of choice for the diagnosis of breast lesions. The transition from open to percutaneous techniques as the favored approach in this setting began in the mid-1990s and was complete by January 2005, at the time of the second international consensus conference on the diagnosis and management of image-detected breast cancer, organized by Silverstein.1
Percutaneous diagnosis is the optimal strategy for planning the management of malignant lesions. The open biopsy of the past has several disadvantages: (1) it usually requires an additional operating room procedure for breast cancer patients; (2) it yields unclear resection margins about twice as often as resection after percutaneous diagnosis; (3) it can complicate or even compromise oncoplastic surgical planning; and (4) it can eliminate options for neoadjuvant treatment.
With percutaneous diagnosis, both palpable and nonpalpable benign lesions may be left in place if the pathology results fully explain the imaged findings. For patients with benign breast biopsy results, representing approximately 80% of breast biopsies, the percutaneous route avoids the expense, distress, and morbidity of an open operation. For the informed patient who requests that a mass with benign features be removed, percutaneous excisional biopsy is ideal as well.
The vast majority of breast cancers and benign breast lesions can be diagnosed accurately with percutaneous large-core needle biopsy.2 Since the goal is diagnosis rather than therapy, surgeons performing image-guided biopsies usually leave most of the target lesion in place; any cancers found are later removed by lumpectomy. Percutaneous management of benign lesions (sampling or excisional biopsy) has several benefits. The patient is spared significant physical and emotional trauma as well as considerable cost. In addition, precious operating room time is reserved for the patients who need it most, enhancing the efficient use of hospital facilities and improving the care of those patients. It is important to note that the efficiency of percutaneous diagnosis can be nullified by the subsequent open surgical removal of a benign lesion already diagnosed by image-guided core biopsy. In retrospect, the surgeon, the patient, and her health insurance provider regret that the lesion was not removed with excisional biopsy (percutaneous or open) in the first place.
In certain circumstances it is critically important to proceed with open excision after image-guided percutaneous biopsy yields benign findings. The most common and the most important reason that benign lesions are removed after percutaneous core biopsy is a lack of concordance between breast imaging findings and the histopathology report. To avoid excision, the surgeon must be satisfied that the findings reported by the pathologist fully explain those on mammographic, sonographic, and physical examination. This rule should be followed without exception to avoid a false-negative result, since a missed diagnosis of breast cancer is unacceptable. In many cases, the experienced breast surgeon can weigh the pathologic findings in light of imaging results on mammography and sonography and determine whether they agree. Any doubt should spur a discussion with the pathologist, the consulting radiologist, or both until a firm conclusion has been reached. Holding clinical–pathologic correlation conferences at regular intervals improves safety and the efficiency of care in any breast care program.
For example, on occasion the surgeon may be surprised to hear from the pathologist that a discrete lesion seen on ultrasound of the breast can be fully accounted for by some form of benign sclerosing adenosis. In other cases, the pathologist will doubt the ability of the histologic findings to explain the imaged abnormality. In the latter case, the lesion must either be excised or an attempt can be made at percutaneous biopsy again to obtain concordant results. Thus the surgeon must work cooperatively with the pathologist and radiologist to prevent false-negative findings in a variety of situations involving a lack of concordance.
While available technology permits almost all lesions to be adequately sampled, and many selected lesions to be removed (by percutaneous excisional biopsy), the surgeon must select the most appropriate instrumentation and biopsy type for each case. Sampling biopsy is ideal for suspicious lesions that are large enough to be targeted with certainty. The residual imaged signature of the lesion can then be used to precisely guide the ultimate removal of a malignant process with intraoperative ultrasound or mammographic needle localization.
Although traditional 14-gauge devices can be used to achieve accurate histologic diagnosis, radiologists, surgeons, pathologists, and oncologists agree that more substantial specimens obtained with the larger, vacuum-assisted/rotational core devices offer advantages. In the first place, upgrades in diagnosis (from atypical hyperplasia to cancer or from ductal carcinoma in situ [DCIS] to invasive cancer) and discordance between imaging and histology are reduced when more tissue is obtained at image-guided core biopsy.2,3 Furthermore, in the modern era, much depends on accurate biological characterization of the individual breast cancer, including hormone receptor status, genomic profile, and protein expression. The initial tissue sampling event can provide the best opportunity to obtain this key biological information for 2 reasons: first, tissue preservation can be optimized; and second, in selected cases neoadjuvant treatment permits alterations in tumor size and biology to guide further treatment and to help the surgeon predict outcome.
The choice of device is also important when percutaneous excisional biopsy is planned. Devices that remove a very large single-core specimen are most suitable when pathologic examination of the entire intact lesion is desirable. Devices that permit the percutaneous multi-core excision of even larger lesions are ideal when the patient wants a larger lesion removed entirely even though the diagnosis is expected to be benign.
Suspicious lesions (Breast Imaging-Reporting and Data System [BI-RADS] category 4 or 5)4 that are visible only on mammography are best approached with stereotactic biopsy (Fig. 33-1). Clustered microcalcifications comprise the vast majority of these ultrasound-invisible lesions. Mass lesions and architectural distortions seen more clearly on mammography than on ultrasound also call for stereotactic biopsy.
Figure 33-1
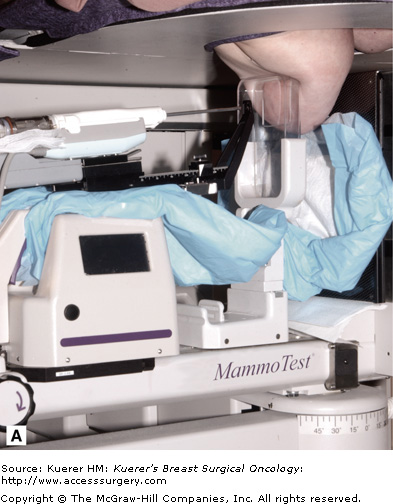

Modern stereotactic biopsy employs a hinged arm that allows images to be taken from −15 degrees and +15 degrees, producing a “stereo” pair of images. The platform provides a mount that will accommodate a broad variety of biopsy devices (A). The prone position stabilizes the patient and is well tolerated (B).(Courtesy of SenoRx, Irvine, California [A], and Ethicon Endo-Surgery, Cincinnati, Ohio [B].)
Modern stereotactic biopsy technique employs the use of a directional vacuum-assisted/rotational core device such as the Mammotome Breast Biopsy System (Ethicon Endo-Surgery, Cincinnati, Ohio), the Automated Tissue Excision and Collection (ATEC) Breast Biopsy and Excision System (Suros Surgical Systems, Indianapolis, Indiana), or a large single-core device such as the Site Select Stereotactic Breast Biopsy System (Site Select Medical Technologies, Pharr, Texas; Fig. 33-2), the Intact Breast Biopsy System (Intact Medical Corporation, Natick, Massachusetts; Fig. 33-3), or the Halo Breast Biopsy Device (Rubicor Medical, Redwood City, California; Fig. 33-4). Understanding similarities and differences in available technology enables the surgeon to select the most appropriate device and application.
Figure 33-2
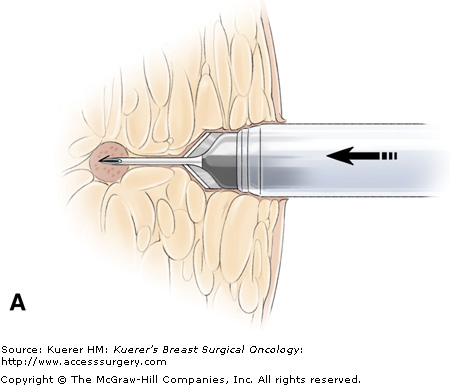
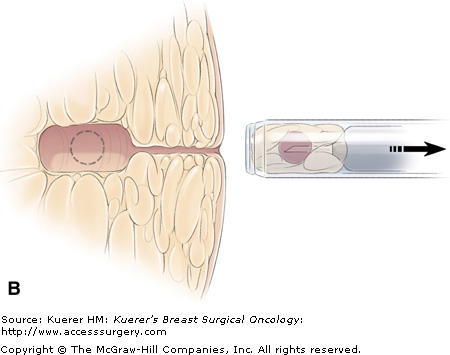
A flat blade at the tip of the Site Select allows the operator to advance the device to the target without removing intervening tissue. The coring cylinder is then advanced to capture the targeted lesion. (Main photograph and drawing from which inset was adapted courtesy of Site Select Medical Technologies, Pharr, Texas.)
Figure 33-3
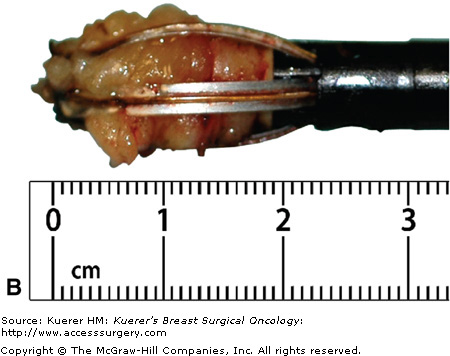
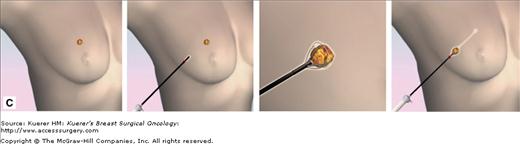
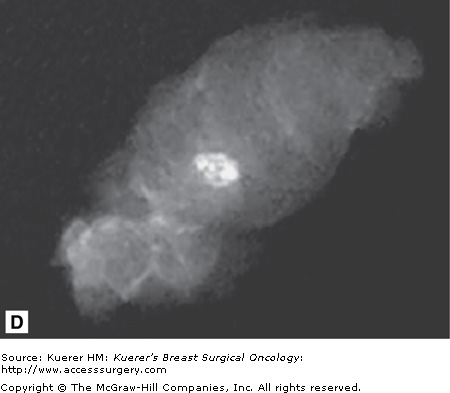
A flat blade (A) in the Intact Breast Lesion Excision System allows the device to be advanced to the targeted position, where the capture basket (B) is deployed, cutting through tissue with radiofrequency current (C). The mammograph (D) reveals the target lesion within the specimen. (Courtesy of Intact Medical Corporation, Natick, Massachusetts.)
Concordance confirmation for stereotactic biopsy of calcifications is straightforward and rests on three key safeguards. First, specimen x-ray of the core samples must show the targeted calcifications convincingly. Next, the image of the biopsy site must demonstrate the removal of a representative portion of the target calcifications. Finally, the pathology report must note the presence of calcifications in the processed tissue; if not, recuts should be requested. Occasionally the target calcifications are composed of calcium oxalate, which can be seen only under polarized light.
When stereotactic biopsy is used for mass lesions or architectural distortions, the surgeon must take greater care than usual to ensure that the targeted lesion is adequately represented in the core samples. Specimen x-rays are usually less helpful in such cases. Careful comparison of prebiopsy and postbiopsy mammograms, however, will confirm that a representative portion of the lesion has been sampled. Discussion with the pathologist and radiologist further establishes concordance. If doubt persists, excision is the rule.
When a lesion is seen clearly on ultrasound, most surgeons with the requisite skills prefer to use ultrasound guidance for sampling.
At least 2 advantages pertain to ultrasound guidance over the stereotactic approach. First, the patient is more comfortable supine than in the prone compression required for stereotactic guidance. Second, sonography is more likely than stereotactic equipment to be available in the surgeon’s clinic. Using the equipment at hand minimizes expense, scheduling delays, and patient inconvenience.
Benign solid lesions tend to have characteristic features on ultrasound evaluation. Analyzing these features affords the surgeon an opportunity to determine whether the pathologic findings are in accord with the imaging features. This analysis is not intended to help determine whether to biopsy a BI-RADS 4 or 5 lesion; biopsy for such lesions is indicated by definition.
The 4 fundamental features that contribute to the reliable analysis of most lesions are the margins, internal echo pattern, shadowing, and height versus width of the mass (Table 33-1). Smooth, well-defined margins suggest that a lesion is benign, whereas irregular, vague margins suggest malignancy. A homogeneous internal echo pattern implies a benign diagnosis, while internal heterogeneity is seen in most cancers. Bilateral corner shadows are consistent with a smooth-walled benign lesion; any other shadow pattern is suspicious. Enhanced transmission of sound through the lesion (causing a brighter echo pattern beneath the lesion than in adjacent tissue) is relatively reassuring, since it results from homogeneous tissue within the mass. Finally, benign lesions tend to be wider than they are tall (width greater than anteroposterior diameter). In contrast, cancers tend to violate the natural tissue planes and to grow taller than they are wide.
| Characteristic | Likely Diagnosis Benign | Malignant |
|---|---|---|
| Margins | Smooth, well defined | Irregular, vague margins |
| Internal echo pattern | Homogeneous | Heterogeneous |
| Shadowing | Bilateral | Nonbilateral |
| Sound transmission through lesion | Enhanced | Attenuated |
| Proportion of width to anteroposterior diameter | Greater | Less |
Breast ultrasound requires the use of a transducer of 7.5 MHz or greater. Although a full description of diagnostic ultrasound techniques is beyond the scope of this chapter, 4 fundamental targeting techniques deserve mention: “skiing,” “painting,” transducer–device alignment, and confirmation scanning.
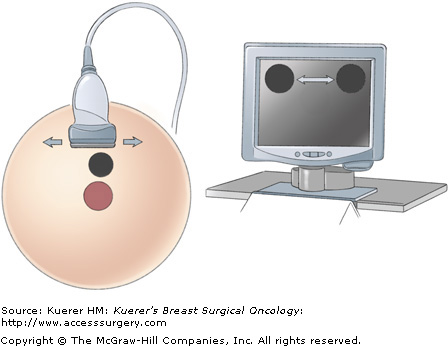
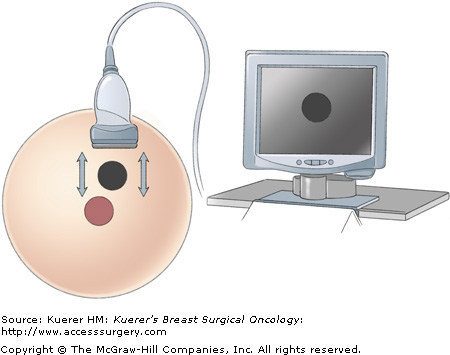
With the lesion in view, the surgeon, sliding the transducer footplate in line with its long axis, performs the “skiing” motion, which moves the image of the lesion from side to side on the monitor (Fig. 33-5). This motion allows the surgeon to place the lesion image in an area of the screen that will be optimal for biopsy access. The “painting” motion, in which the transducer footplate is moved perpendicular to its long axis, brings the lesion into and out of view at a given position on the monitor, permitting the lesion’s widest portion to be targeted for the best sample (Fig. 33-6).
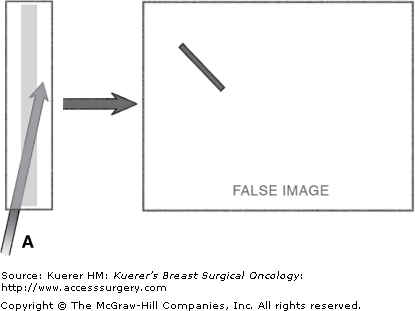
The most common error made by surgeons who are gaining skill in ultrasound device guidance is failure to align the device with the transducer (Fig. 33-7). Small alignment deviations result in failure to visualize the tip of the device and to be able to anticipate the trajectory of the advancing tip. Frequent direct visual checks of device–transducer alignment will help to prevent this dangerous situation. Further alignment is required if the full length of the device is not well seen.