Hypothyroidism in Infants, Children, and Adolescents: Acquired Hypothyroidism
Guy Van Vliet
Johnny Deladoëy
The unique feature of hypothyroidism in infants, children, or adolescents is that, if the diagnosis is delayed, the condition may have a profound impact on skeletal growth and maturation, pubertal development, and adult height. When it occurs before birth, hypothyroidism may retard skeletal maturation, but it does not result in stunted intrauterine growth. However, starting immediately after birth (1,2), and until epiphyseal fusion, longitudinal bone growth is exquisitely sensitive to thyroid hormone deficiency. Thus, slowing of linear growth is the most sensitive clinical indicator of hypothyroidism in children. On the other hand, longstanding hypothyroidism appearing in infancy may still have permanent effects on later cognitive development (3), while when it happens after age 3 years, it no longer has this dramatic potential consequence. In fact, it is the initiation of treatment of severe hypothyroidism acquired during childhood and adolescence that may transiently decrease learning ability and alter behavior, sometimes dramatically (4).
Epidemiology and Causes of Acquired Hypothyroidism in Children
The causes of acquired hypothyroidism in children and adolescents are shown in Table 54C.1. In addition to these disorders, in some infants with congenital hypothyroidism the onset of symptoms and signs of hypothyroidism may be delayed, and affected children may therefore present as if they have acquired hypothyroidism. This is most common in children with inborn errors of thyroid hormone biosynthesis (see Chapter 36), but the mechanism of the delayed expression of the gene defect is unknown (5), although an abundant neonatal iodine supply has been reported to mask the hypothyroidism due to DEHAL1 mutations (6). Children with abnormal peripheral thyroid hormone metabolism from mutations in MCT8 (7) or in SECISBP2 (8) typically have mild abnormalities of thyroid function tests
and are therefore not identified by neonatal screening for congenital hypothyroidism (see Chapter 58). On the other hand, children with central hypothyroidism caused by mutations in the β-subunit of thyrotropin (TSH) are missed by TSH-based neonatal screening programs and may only be recognized later.
and are therefore not identified by neonatal screening for congenital hypothyroidism (see Chapter 58). On the other hand, children with central hypothyroidism caused by mutations in the β-subunit of thyrotropin (TSH) are missed by TSH-based neonatal screening programs and may only be recognized later.
Table 54C.1 Causes of Acquired Hypothyroidism in Children and Adolescents | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
There are few studies of the prevalence of acquired hypothyroidism in children. In a study of 103,500 people aged 0 to 22 years in Scotland, 140 patients (0.14%) had received prescriptions for thyroxine (T4) on a regular basis (9). The male to female ratio was 1:2.8. A review of these patients’ charts revealed that 75% had acquired hypothyroidism. The vast majority had primary hypothyroidism and evidence of autoimmune thyroid disease, 3.5% had type 1 diabetes mellitus, 2% had juvenile rheumatoid arthritis, and 1.5% had Down’s syndrome. Geographic variations in the prevalence of hypothyroidism caused by autoimmune thyroid disease suggest that iodine deficiency may be protective (10). Indeed, Hashimoto’s thyroiditis is a common diagnosis in pediatric endocrine clinics in iodine-replete North America, but is much rarer in European countries such as Belgium, in which iodine intake is much lower.
Less common than hypothyroidism due to autoimmune thyroid disease is radiation-induced hypothyroidism. This may occur as a result of external radiation therapy for tumors of the head and neck, or after radioiodine therapy for thyrotoxicosis (see Chapter 32). Hypothyroidism after thyroidectomy is rare in children, because few children undergo thyroid surgery. Hypothyroidism due to drugs is also rare. Interferon-α, lithium, and iodine-containing drugs can cause hypothyroidism in children, as in adults, primarily in children with preexisting chronic autoimmune thyroiditis (see Chapter 11B).
An exceptional cause of hypothyroidism is consumptive hypothyroidism, resulting from the overexpression of type 3 deiodinase by large liver or cutaneous hemangiomas (11). Although some of these infants have hypothyroidism at birth (12), most are present in the first few months of life, when hemangiomas are typically at their maximal size. Importantly, severe primary hypothyroidism requiring a high dose of T4 was the presenting manifestation of multiple hepatic hemangiomas in one child, suggesting that a search for these tumors should be undertaken in any child with unexplained primary hypothyroidism who requires a high dose of T4 (13).
Lastly, iodine deficiency remains an important cause of hypothyroidism worldwide (see section on iodine deficiency in Chapter 11D). Although acquired hypothyroidism due to iodine deficiency is rare in North America and other countries where iodized salt is available, it can occur in children whose diets are severely restricted in salt and iodine-containing foods (14).
Acquired central hypothyroidism is typically associated with other pituitary hormone deficiencies. It is rarely the first or presenting manifestation of hypothalamic or pituitary disease, including tumors and infiltrative diseases such as histiocytosis, or of developmental defects with delayed expression.
Clinical Presentation
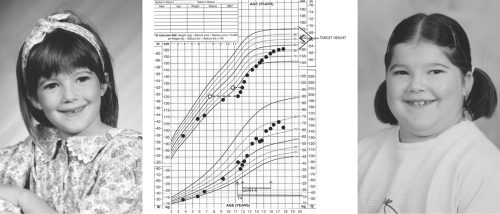
The symptoms and signs of acquired hypothyroidism in children and adolescents depend on its severity and duration. Some patients present with a relatively short history of fatigue, constipation, dry skin, and cold intolerance, without a change in their growth pattern and without retardation of bone maturation, reflecting a sudden onset of hypothyroidism. However, in the vast majority of patients, assessment of growth reveals long-standing hypothyroidism. Typically, there is a rather abrupt deceleration in height gain at some time in the more or less distant past, whereas weight gain is relatively preserved. Thus, the patients present with short stature and relative weight excess, reflecting myxedema and not increased adiposity (Fig. 54C.1). This growth pattern is fundamentally different from that seen in children with exogenous obesity, who have accelerated rates of linear growth; in such a situation, there is no reason to measure serum TSH but this is often done and occasionally shows mildly elevated levels that normalize with weight loss and do not, therefore, require thyroxine treatment (15). The effect of hypothyroidism on bone maturation is even more pronounced than its effect on linear growth. Tooth eruption is also delayed. Other skeletal effects of hypothyroidism in children include stippling of the epiphyses and slipped capital femoral epiphyses (16).
Because longstanding hypothyroidism retards bone maturation, it is often assumed that it invariably retards pubertal development. However, some children with severe primary hypothyroidism have sexual precocity (17): This rare occurrence appears to result from activation of follicle-stimulating hormone receptors by the very high serum TSH concentrations. Accordingly, affected boys have testicular enlargement without testosterone secretion (18), and affected girls have functional ovarian cysts with vaginal bleeding, either with no other signs of puberty or with breast development and galactorrhea but no pubic hair (17,19). The reason why all children with longstanding primary hypothyroidism do not develop this syndrome is unclear since individual polymorphisms in the FSH receptor do not affect its response to TSH (20).
Hypothyroidism acquired after 3 years of age (the critical period during which it has an irreversible effect on brain development) does not typically affect school performance
and progression. If the hypothyroidism is severe and of long duration, the child may require more time to accomplish the assigned tasks, but will rarely be held back academically. In fact, more often, school performance deteriorates after treatment is started, as is commonly observed in children with hyperthyroidism.
and progression. If the hypothyroidism is severe and of long duration, the child may require more time to accomplish the assigned tasks, but will rarely be held back academically. In fact, more often, school performance deteriorates after treatment is started, as is commonly observed in children with hyperthyroidism.
Aside from its effects on growth, maturation, and puberty, the symptoms and signs of acquired hypothyroidism in children and adolescents are similar to those in adults (see Chapter 39). Thus, a constellation of increasing fatigue, constipation, cold intolerance, and puffiness of the face should alert the clinician to the possibility of hypothyroidism. Hypothyroidism has also been reported in otherwise asymptomatic children with hypercholesterolemia, and serum TSH should be measured in children with any type of hyperlipidemia (21).
In children with any symptom or sign of hypothyroidism, the size of the thyroid should be determined. A goiter is present when the thyroid lobes are larger than the distal phalanx of the thumb of the child; this “individual calibrator” may seem rather crude but is useful in children given their greatly varying size. In iodine-sufficient areas, the most common cause of goiter associated with hypothyroidism is Hashimoto’s thyroiditis, in which case both lobes of the thyroid are usually moderately enlarged, firm, and irregular (“pebbly”). Nodules greater than 1 cm in diameter are rare, but a “Delphian node” is a frequent finding (a lymph node sometimes as small as a grain of rice that can be palpated above the isthmus, close to the midline). A family history of autoimmune thyroid disease is often elicited. Although a goiter is often present at the time of diagnosis of Hashimoto’s thyroiditis, the gland will usually atrophy over time. Thyroid atrophy may be present at diagnosis at any age, but this appears to be more common in infants (22). If the thyroid is not palpated in its normal location, the throat should be carefully examined, because some children with large lingual thyroids may be missed at neonatal screening and present at later ages with mild hypothyroidism (23).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree