Hypothyroidism in Infants and Children: Congenital Hypothyroidism
Guy Van Vliet
Johnny Deladoëy
A relationship between an absent or defective thyroid gland and mental retardation was recognized long ago, but the most striking description of the clinical picture of “cretinism,” as it was then called, was made by Sir William Osler, who in 1897 wrote that “No type of human transformation is more distressing to look at than an aggravated case of cretinism.” The stunted stature, the semi-bestial aspect, the blubber lips, retroussé nose sunken at the root, the wide open mouth, the lolling tongue, the small eyes half-closed with swollen lids, the stolid, expressionless face, the squat figure, the muddy dry skin, combine to make the picture of what has been termed “pariah of nature” (1).
A century ago, thyroid extract was found to normalize the physical appearance of patients with congenital hypothyroidism (CH). However, the mental deficiency was irreversible, because treatment was started after the most critical period of sensitivity of the brain to thyroid hormone deficiency (2). Mental deficiency from CH was only eradicated when widespread mass biochemical screening of neonates was implemented starting in the 1970s (see Chapter 54A). During the same period, progress toward the elimination of iodine deficiency, the most important extrinsic factor causing CH, has been made in many countries (see Chapter 11D).
Classification of Congenital Hypothyroidism
Like acquired hypothyroidism, CH can be classified as primary (thyroidal), if the defect occurs at the level of the thyroid gland; peripheral, if the defect is in thyroid hormone sensitive peripheral tissues; and central, if the defect involves the hypothalamus or pituitary gland (Table 54B.1). Central hypothyroidism may be further divided into secondary (pituitary causes) and tertiary (hypothalamic causes). Hypothyroidism can also be classified according to whether it is transient or permanent. Permanent primary CH is the most common form of CH, and is in fact the most common congenital endocrine disorder: Estimates of its prevalence depend on the screening methods, algorithms and cut-offs used but average 1 in 2,500 newborn infants (see Chapter 54A).
Table 54B.1 Classification of Congenital Hypothyroidism | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In about two-thirds of infants, permanent primary CH is caused by thyroid dysgenesis, which includes multiple abnormalities in thyroid gland development. Among them, the most common is ectopic thyroid tissue (∼50% of cases). The ectopic thyroid tissue is usually located above where the normal thyroid should be, as a result of an arrest in downward migration of the median thyroid anlage during embryonic development; this ectopic tissue contains the only detectable thyroid follicular cells in the affected infants (see Chapter 2). The hypothyroidism observed in most patients with ectopic thyroid likely results from a smaller amount of tissue (ectopic thyroids lack lateral lobes) and from a limitation to thyrotropin (TSH)-dependent compensatory growth (3). Approximately 15% of infants with CH have complete absence of thyroid tissue (athyreosis); this may result from a defect in the differentiation of thyroid follicular cells or from their disappearance during prenatal life. Less common are thyroid hypoplasia (<5% of cases), which is characterized by small thyroid lobes in the normal position, and thyroid hemiagenesis (<1% of cases), in which one lobe (usually the left), is missing. Lastly, about 10% of cases of permanent primary CH are due to defects in thyroid hormone biosynthesis (thyroid dyshormonogenesis), which, because of the trophic actions of TSH, eventually lead to thyroid enlargement. However, thyroid enlargement is almost never detected clinically at birth. Lastly, lowering TSH screening cut-offs leads to identifying additional cases with normal thyroid anatomy and thus mild functional disorders from unknown mechanisms and of uncertain impact on cognitive development (4).
Permanent central hypothyroidism, resulting from defects at the level of the hypothalamus or pituitary, occurs in less than 1 in 50,000 births and is almost always associated with clinically identifiable deficiencies of other pituitary hormones. Peripheral hypothyroidism from resistance to thyroid hormone or from abnormal thyroid hormone metabolism (see Chapter 58) is even more uncommon. In addition to these permanent conditions, transient alterations in thyroid function are occasionally encountered.
Permanent central hypothyroidism, resulting from defects at the level of the hypothalamus or pituitary, occurs in less than 1 in 50,000 births and is almost always associated with clinically identifiable deficiencies of other pituitary hormones. Peripheral hypothyroidism from resistance to thyroid hormone or from abnormal thyroid hormone metabolism (see Chapter 58) is even more uncommon. In addition to these permanent conditions, transient alterations in thyroid function are occasionally encountered.
Etiology and Pathogenesis
Permanent Congenital Hypothyroidism
Thyroid dysgenesis was until recently considered a sporadic entity, although there had been some reports of familial occurrence. A systematic evaluation of the heritability of thyroid dysgenesis in France revealed that 48 of 2,472 infants with thyroid dysgenesis (2%) had an affected relative; this figure is 15-fold higher than predicted by chance alone. Pedigree analysis was most compatible with dominant inheritance with variable penetrance, although there was genetic heterogeneity and possible multigenic inheritance in some pedigrees (5). In another study by the same group, subclinical abnormalities of thyroid development (mostly persistence of thyroglossal duct remnants) were identified by ultrasonography in 8% of euthyroid first-degree relatives of children with thyroid dysgenesis, as compared with only 0.8% in a control population (6). By contrast, a standard method for estimating heritability of disease, the systematic survey of monozygotic twins, revealed that discordance for thyroid dysgenesis was an almost constant finding (>95%) [(7) and personal observations]. Also incompatible with simple Mendelian inheritance is the marked female predominance seen in thyroid dysgenesis (especially thyroid ectopy) (8).
Consistent with these epidemiologic findings, germline mutations in genes known to be involved in thyroid development (NKX2.1 or TTF-1, FOXE1 or TTF-2, PAX-8, GLIS3) and growth (TSH receptor) are identified in at most 2% of patients with sporadic thyroid dysgenesis (see Chapter 2). Other genes may be involved (9) but the most logical conclusion from the above is that non-Mendelian mechanisms explain most cases of thyroid dysgenesis. These include epigenetic modifications, somatic mutations occurring early in embryogenesis in the thyroid bud, or stochastic developmental events. A two-hit model combining germline and somatic (epi)genetic variation is discussed in detail elsewhere (10).
Thyroid dyshormonogenesis results from a defect in any one of the steps involved in the biosynthesis of thyroid hormone, from the transport of iodine across the apical membrane to its intracellular recycling from mono- and di-iodotyrosines. These defects are inherited as autosomal recessive traits and occur at higher frequency in consanguineous families. In population-based studies, mutations inactivating the thyroperoxidase gene seem to be the most commonly involved (see Chapter 36).
Given the different mechanisms underlying CH caused by thyroid dysgenesis and by dyshormonogenesis, it is essential that these two forms be distinguished when comparing prevalence figures between ethnic groups. When this is done, it appears that thyroid dysgenesis, but not dyshormonogenesis, is less common in Blacks than in Caucasians (11). The lack of consistent seasonal variation in the prevalence of thyroid dysgenesis argues against a role of viral infections in the mother (12). The stable prevalence over two decades (4) contrasts with the decline in heart (13) and neural (14) defects after the introduction of maternal folate supplementation in Canada.
Congenital central hypothyroidism is usually caused by developmental or functional abnormalities of the pituitary or hypothalamus (Table 54B.1). Isolated central hypothyroidism caused by mutations of the gene for the β-subunit of TSH is exceedingly rare, with only 15 cases described. These mutations result in the production of a TSH molecule with little biologic or immunologic activity, and affected infants usually have severe hypothyroidism (15,16). Other rare cases are due to inactivating mutations in the thyrotropin-releasing hormone (TRH) receptor (17,18), in pituitary transcription factors (19), or to unknown mechanisms. TSH screening misses infants with these rare disorders, but this has occurred in only 3 of 1.7 million infants in Québec over 20 years.
Transient Congenital Hypothyroidism
Transient primary CH has been well described in areas where the iodine intake of the population is either severely or moderately deficient (see Chapter 11D). In the latter areas, it usually occurs in premature newborns and is associated with an acute iodine overload, most often from the use of iodine-containing antiseptic agents in the infant or mother. Cessation of the use of these antiseptic agents in newborn nurseries has led to the
almost complete disappearance of this form of transient primary hypothyroidism (20). It is rare in areas of iodine sufficiency such as North America (21).
almost complete disappearance of this form of transient primary hypothyroidism (20). It is rare in areas of iodine sufficiency such as North America (21).
In iodine-sufficient areas, the most common cause of transient primary hypothyroidism is maternal therapy with an antithyroid drug. These drugs are cleared rapidly from the infant’s circulation, and therefore the hypothyroidism is short-lived; most affected infants have normal serum TSH and free thyroxine (T4) concentrations at recall. Transplacental transfer of maternal antibodies that block the action of TSH is a rare cause of transient primary hypothyroidism in newborn infants, causing only about 1% to 2% of cases (22); these infants may still have hypothyroidism at the time of recall because the antibodies are cleared relatively slowly. These antibodies do not interfere with the formation, migration, and growth of the thyroid gland, and therefore do not cause permanent primary CH. Transient primary CH from either heterozygous (23) or homozygous (24) mutations in DUOX2, the gene encoding one of the enzymes involved in the generation of H2O2 needed for iodine oxidation in the thyroid, may be more common than initially thought. Lastly, transient central hypothyroidism has also been reported in infants whose mothers had uncontrolled hyperthyroidism during pregnancy (25).
Clinical Presentation
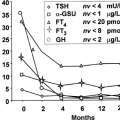
Few infants with CH have an abnormal appearance or behavior at birth or even at recall when the diagnosis is confirmed and treatment started. On the other hand, the few newborn infants with clinical manifestations of hypothyroidism would probably benefit from diagnosis in the first days after birth and from treatment begun even earlier than can be achieved through screening programs. Thus, it remains important to recognize the clinical manifestations of hypothyroidism in newborn infants. Unexplained postmaturity and macrosomia with an open posterior fontanel can be recognized on the day of birth (26). Very few infants have a clinically detectable goiter, and in even fewer is it of sufficient size to impede neck flexion during delivery or to cause airway obstruction (27). Other symptoms and signs include poor feeding, hypothermia, lethargy, constipation, prolonged jaundice, abdominal distention, umbilical hernia, dry and mottled skin, macroglossia, a hoarse cry, and a myxedematous appearance (Fig. 54B.1).
A clinical suspicion of hypothyroidism in a newborn or very young infant should always lead to the immediate measurement of serum TSH and free T4, even if the screening blood sample has already been collected. Also, the possibility of human or technical error in the screening process, of central hypothyroidism [in areas using a TSH-first screening approach (see Chapter 54A)], or of falsely normal (false-negative) results should be kept in mind.
Among infants referred for an abnormal screening result, a family history should be obtained, with focus on consanguinity and on the existence of even distant relatives with CH (Table 54B.2). A family history of thyroid disorders with onset in later life is usually irrelevant, but a maternal history of thyroid disease, treatment for thyroid disease, or exposure to iodine-rich compounds such as radiographic contrast agents is relevant. An examination of the infant seeking the above-listed physical signs should be conducted. A goiter may be seen or palpated when the infant’s neck is hyperextended. The only extrathyroid malformations consistently associated with thyroid dysgenesis
are defects in heart septation (8,28,29). These are usually mild and resolve spontaneously: In the absence of signs or symptoms, they do not need to be screened for.
are defects in heart septation (8,28,29). These are usually mild and resolve spontaneously: In the absence of signs or symptoms, they do not need to be screened for.
Table 54B.2 Diagnostic Evaluation of a Newborn Infant Suspected to Have Congenital Hypothyroidism | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree