Objectives
- 1.
Discuss the embryology and anatomy of the pituitary gland.
- 2.
Review the function of the neurohypophysis (posterior pituitary), including the synthesis, regulation, and function of two neurohormones: antidiuretic hormone (ADH; also called vasopressin ) and oxytocin.
- 3.
Describe the neurovascular connection between the hypothalamus and the adenohypophysis (anterior pituitary).
- 4.
Develop the concept of an endocrine axis.
- 5.
Describe the cytology of the adenohypophysis, along with the structure and function of the six hormones produced by the adenohypophysis.
- 6.
Discuss the significant direct effects of growth hormone and prolactin on nonendocrine organs.
- 7.
Present some forms of pituitary pathophysiology.
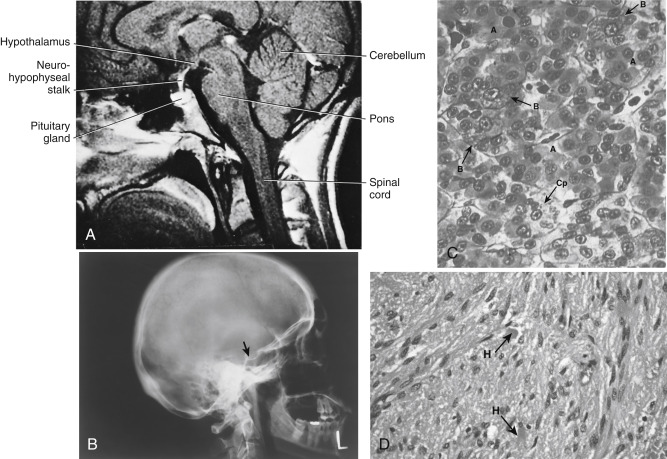
The pituitary gland (also called the hypophysis ) is a small (about 0.5 g in weight) yet complex endocrine structure at the base of the forebrain ( Fig. 5.1A and B ). It is composed of an epithelial component, called the adenohypophysis or anterior pituitary , and a neural structure, called the neurohypophysis . The caudal end of the neurohypophysis is called the pars nervosa, or posterior pituitary. The anterior pituitary contains five cell types that secrete six hormones. The neurohypophysis acts as a site of release of multiple neurohormones. All endocrine functions of the pituitary gland are regulated by the hypothalamus and by negative and positive feedback loops.
Embryology and Anatomy
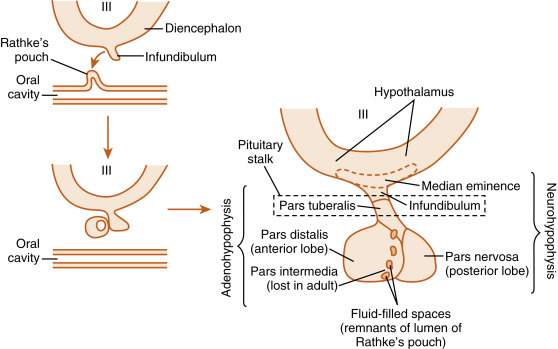
Microscopic examination of the pituitary reveals two distinct types of tissues: epithelial and neural ( Fig. 5.1C and D ). This dual nature of the gland is best understood by reviewing its development. During development, a caudal extension of the primitive forebrain (i.e., the diencephalon) grows toward the roof of the primitive oral cavity ( Fig. 5.2 ). This neural downgrowth, called the infundibulum , secretes factors that induce the epithelium of the roof of the oral cavity to extend cranially toward the base of the developing brain. This extension of the oral ectoderm is called Rathke pouch . As Rathke pouch moves upward, the following events occur:
- 1.
Rathke pouch loses its contact with the oral cavity, and by doing so, becomes a ductless endocrine structure. Remnants of Rathke pouch may persist and can give rise to craniopharyngiomas.
- 2.
Rathke pouch comes into direct contact with the infundibulum. The cells on the posterior side of the pouch lumen facing the infundibulum give rise to the pars intermedia in the fetus. These cells degenerate in the adult human pituitary. The cells on the anterior side of the pouch lumen facing away from the infundibulum expand considerably and give rise to the pars distalis . The pars distalis makes up almost all of the adenohypophysis in the adult and is also referred to as the anterior pituitary . A third division of Rathke pouch develops into the pars tuberalis and is composed of a thin layer of cells that wrap around the infundibular stalk at the superior end of the anterior pituitary. To summarize, the adenohypophysis (i.e., the epithelial portion of the pituitary, or anterior pituitary) develops from epithelial cells (the oral ectoderm) and is composed of the pars distalis, a thin layer called the pars tuberalis , and the pars intermedia, which is lost in adult humans.
- 3.
The infundibular process expands at its lower end to give rise to a structure called the pars nervosa . The pars nervosa is also called the posterior lobe of the pituitary (or simply, the posterior pituitary ). At the superior end of the infundibulum, a funnel-shaped swelling develops called the median eminence . The rest of the infundibular process, which extends from the medium eminence down to the pars nervosa, is called the infundibulum. To summarize, the neurohypophysis develops from a downgrowth of neural tissue at the base of the diencephalon (corresponding to the hypothalamus in the adult) and gives rise to the pars nervosa, the infundibulum, and the median eminence. The infundibulum and the pars tuberalis make up the pituitary stalk .
With development, the pituitary gland becomes encased in the sphenoid bone in a structure called the sella turcica (see Fig. 5.1B ). The pituitary stalk emerges superiorly out of the sella turcica in the vicinity of the optic nerves and optic chiasm. Generally, cancers of the pituitary have only one way to expand, which is superiorly into the brain and against the optic nerves . Thus any increase in the size of the pituitary is often associated with dizziness or vision problems . The sella turcica is sealed off from the brain by a membrane called the diaphragma sellae . Defective development of the diaphragma sellae can allow cerebrospinal fluid to enter the sellar cavity and encroach on developing pituitary tissue. This can give rise to empty sella syndrome, which represents a reduction of pituitary tissue (but not always pituitary function) within the sella turcica.
Neurohypophysis
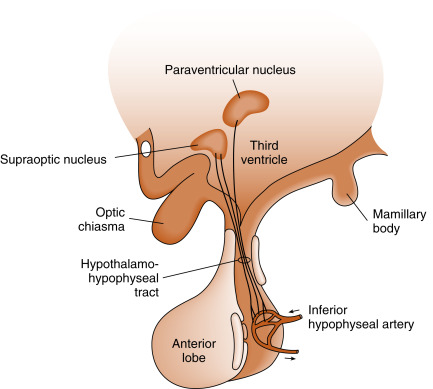
The pars nervosa is a neurovascular structure that is the site of release of neurohormones adjacent to a rich bed of fenestrated capillaries. The peptide hormones that are released are antidiuretic hormone (ADH) (also called vasopressin ) and oxytocin . The cell bodies of the neurons that project to the pars nervosa are located in the supraoptic nuclei (SON) and paraventricular nuclei (PVN) of the hypothalamus (in this context, a nucleus refers to a collection of neuronal cell bodies residing within the central nervous system [CNS]—a ganglion is a collection of neuronal cell bodies residing outside the CNS). The cell bodies of these neurons are described as magnocellular (i.e., large cell bodies) and are equipped with enough biosynthetic capacity to produce a short-lived peptide hormone that is released into and diluted by the peripheral circulation. The magnocellular neurons project axons down the infundibular stalk as the hypothalamohypophyseal tracts . These axons terminate in the pars nervosa ( Fig. 5.3 ). In addition to axonal processes and termini from the SON and PVN, there are glial-like supportive cells called pituicytes . As is typical of endocrine organs, the posterior pituitary is extensively vascularized, and the capillaries are fenestrated, thereby facilitating diffusion of hormones into the vasculature.
Synthesis of Antidiuretic Hormone (Vasopressin) and Oxytocin
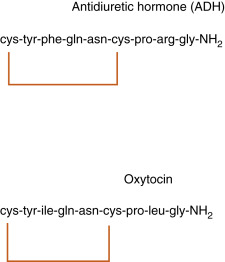
ADH and oxytocin are nonapeptides (9 amino acids) and are similar in structure, differing in only 2 amino acids ( Fig. 5.4 ). They have limited overlapping activity. ADH (vasopressin) and oxytocin are synthesized as preprohormones. Each prohormone harbors the structure of oxytocin or ADH, each of which is composed of 9 amino acids and a cosecreted peptide called either neurophysin-II (associated with ADH) or neurophysin-I (associated with oxytocin). These preprohormones are called preprovasophysin and preprooxyphysin . The N-signal peptide is cleaved while the peptide is transported into the endoplasmic reticulum. The prohormone is packaged in the endoplasmic reticulum and Golgi in a membrane-bound secretory granule in the cell bodies within the SON and PVN ( Fig. 5.5 ). The secretory granules are conveyed intraaxonally through a “fast” (i.e., millimeters/hour) adenosine triphosphate (ATP)–dependent transport mechanism down the infundibular stalk to the axonal termini in the pars nervosa. During transit of the secretory granule, the prohormones are proteolytically cleaved, producing equimolar amounts of hormone and neurophysin. Secretory granules containing fully processed peptides are stored in the axonal termini. Axonal swellings due to the storage of secretory granules can be observed by light microscopy with certain stains and are termed Herring bodies (see Fig. 5.1D ).
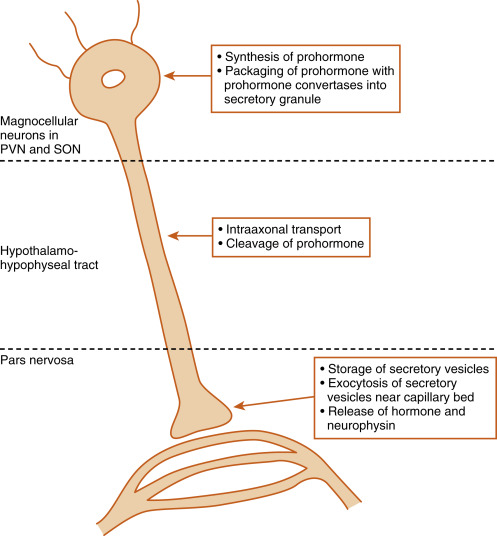
ADH and oxytocin are released at the pars nervosa in response to stimuli that are primarily detected at the cell bodies and their dendrites in the SON and PVN of the hypothalamus. The stimuli are primarily in the form of neurotransmitters released from hypothalamic interneurons. On sufficient stimulus, the neurons will depolarize and propagate an action potential down the axon. At the axonal termini, the action potential increases intracellular Ca 2+ and results in a stimulus-secretion response, with the exocytosis of ADH or oxytocin, along with neurophysins, into the extracellular fluid (ECF) of the pars nervosa (see Fig. 5.5 ). Both hormones and neurophysins gain access to the peripheral circulation, and both can be measured in the blood.
There is no known biologic function for circulating neurophysins. However, several mutations in familial diabetes insipidus (in which ADH production is deficient) have been mapped to mutations within the neurophysin structure, suggesting that the sequence and structure of the neurophysin portion are important for the correct processing of the prohormone.
Because posterior pituitary hormones are synthesized in the hypothalamus rather than the pituitary, hypophysectomy (pituitary removal) does not necessarily permanently disrupt synthesis and secretion of these hormones. Immediately after hypophysectomy, secretion of the hormones decreases. However, over a period of weeks, the severed proximal end of the tract will show histologic modification, and pituicytes will form around the neuron terminals. Secretory vacuoles are visible, and secretion of hormone resumes from this proximal end. Secretion of hormone can actually potentially return to normal levels. In contrast, a lesion higher up on the pituitary stalk can lead to loss of the neuronal cell bodies in the PVN and SON.
Antidiuretic Hormone
Actions of Antidiuretic Hormone
(Mosby Physiology Monograph Series: Chapter 9 in Renal Physiology , 6th Ed., BM Koeppen and BA Stanton)
The primary functions of ADH in humans are maintenance of normal osmolality of body fluids and maintenance of normal blood volume. The primary target cells of the ADH are the cells lining the distal renal tubule and the principal cells of the collecting ducts in the kidney. ADH binds to the vasopressin-2 (V2) receptor on the basal side of renal cells ( Fig. 5.6 ). The V2 receptor is a G-protein–coupled receptor (GPCR) linked to the Gs-cAMP-PKA pathway. Signaling from the V2 receptor induces the insertion of vesicles containing the water channel protein, called aquaporin-2 , into the apical membrane of the principal cells, thereby increasing water permeability of this membrane. ADH also increases the gene expression and new synthesis of aquaporin-2. While the basolateral side of the target cells constitutively expresses aquaporin-3 and -4, the ADH-induced increase in apical membrane aquaporin-2 enhances the transepithelial flow of water from the lumen toward the renal interstitium. Therefore, in the presence of ADH, urine flow decreases ( antidiuresis ), and urine osmolality approaches that of the medullary epithelium (about 1200 mOsm/kg). In the absence of ADH, urine flow increases ( diuresis ), and urine osmolality decreases.
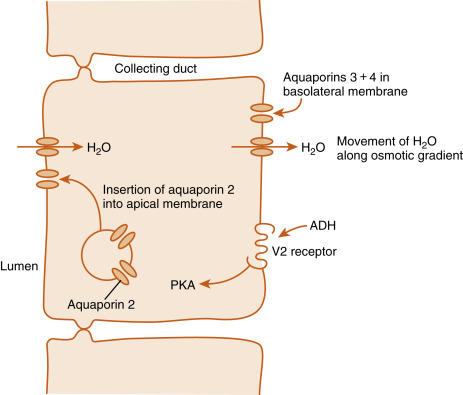
ADH increases mesangial cell contraction, which lowers the filtration coefficient of the glomerular membrane and therefore decreases the glomerular filtration rate. This action will further decrease the volume of urine flow. ADH inhibits renin release, a response that could be beneficial in compensation for an increase in ECF osmolality.
As part of its role in the defense against the cardiovascular consequences of severe volume depletion, ADH levels increase to supraphysiologic levels (i.e., increase by greater than 100-fold) during vasodilatory shock . At these levels, ADH binds to the V1 receptor on vascular smooth muscle. The V1 receptor is coupled to a Gq–phospholipase C-intracellular Ca 2+ signaling pathway, which increases vascular smooth muscle contraction. Thus the vasopressive actions of ADH become important during early states of vasodilatory shock.
Regulation of Antidiuretic Hormone Secretion
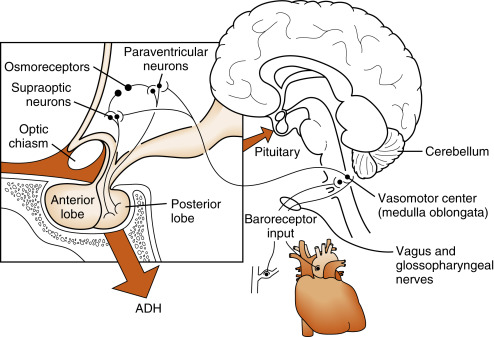
ADH is released in response to increased ECF osmolality or decreased blood volume and pressure. Osmoreceptive neurons , probably in the hypothalamus or circumventricular organs, innervate the magnocellular neurons of the PVN and SON. These osmoreceptive neurons respond to changes in ECF osmolality by shrinkage or swelling. Thus increased osmolarity indirectly stimulates the magnocellular cells and action potential frequency increases in the neuronal axons constituting the hypothalamohypophyseal tract, with a resultant increase in posterior pituitary ADH release. Because the actual stimulus is cellular dehydration, the response to the hyperosmolality depends on the nature of the solutes. Solutes such as sodium, sucrose, and mannitol that do not readily enter the osmoreceptor cells are effective stimulators, whereas urea, to which the cells are more permeable, has about one third the potency of sodium. These effects may be demonstrated with the following relationship:
↑ ECF asmablity → ↑ ADH → ↑ Renal water reabsorption → ↓ ECF asmablity
The regulatory system is sensitive to serum osmolality changes in the range of 280 to 295 mOsm/kg ( Fig. 5.7 ). Within this range, a rise in as little as 1% in serum osmolality will stimulate a measurable increase in ADH secretion.
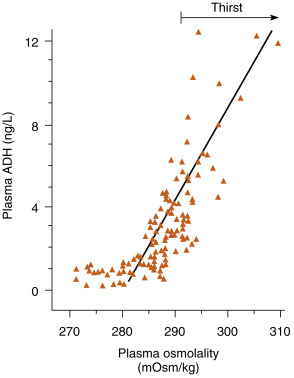
ADH release can also be stimulated by a drop in effective blood volume. The receptors for this stimulus are the cardiovascular volume receptors , including low-pressure receptors in the atria of the heart, great veins, and pulmonary vasculature and high-pressure receptors in the aortic arch and carotid sinus baroreceptors ( Fig. 5.8 ). Although all of these volume receptors are capable of regulating ADH secretion, the predominant regulator appears to be the atrial volume receptors. The sensitivity of the system to volume change is low at small volume changes. However, volume change does become a significant stimulus when circulating blood volume decreases 8% to 10% or more ( Fig. 5.9 ). This becomes the only mechanism of ADH stimulation during hemorrhage. A decrease in effective blood volume increases the sensitivity of ADH secretion to an increase in ECF osmolality.
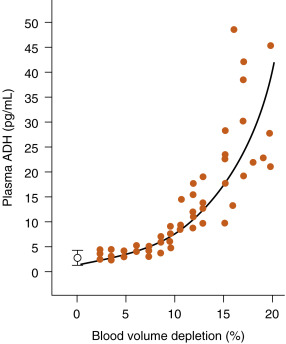
Relationship Between Osmotic and Volume Stimuli
Vascular volume influences the sensitivity of the system to osmotic stimuli. At lower vascular volumes, the system becomes more sensitive to a rise in serum osmolality. In turn, as vascular volume increases, the sensitivity of ADH release to osmotic stimuli decreases.
Other Factors Altering Antidiuretic Hormone Secretion
Several drugs, including barbiturates, nicotine, and opiates, increase ADH secretion. Alcohol is an effective suppressor of ADH secretion. For this reason, consumption of alcoholic beverages can lead to dehydration rather than volume expansion. Nausea increases ADH secretion, affording a protective effect against imminent volume loss due to vomiting. The hormones atrial natriuretic peptide (ANP) and cortisol (see Chapter 7 ) inhibit ADH secretion.
Regulation of Thirst
The regulation of thirst and drinking behavior is an important component of body fluid balance regulation. Thirst is regulated by many of the same factors that regulate ADH secretion. Increased serum osmolality, decreased vascular volume, and ADH secretion are effective stimuli for thirst. The osmoreceptors regulating thirst involve medial hypothalamic regions that approximate the osmoreceptors regulating ADH secretion. Angiotensin II is also thought to play a major role in the regulation of thirst. There are many components to the regulation of drinking, which include, in humans, chemical factors, social factors, and pharyngeal and gastrointestinal factors.
Degradation
ADH is predominantly destroyed by proteolysis in the kidney and liver. The circulating half-life of ADH is about 15 to 20 minutes.
A deficiency in ADH production results in diabetes insipidus (DI) . People with DI are unable to concentrate urine normally and, therefore, excrete a large volume of urine. These individuals can have urinary flow rates as high as 25 L/day. Thirst increases as a result of the dehydration caused by the high urinary flow. Diabetes insipidus differs from osmotic diuresis in that in the former, the urinary osmolality (or specific gravity) is much lower than plasma, whereas in the latter, the urinary osmolality approaches that of plasma.
Neurogenic (Pituitary-Hypothalamic) Diabetes Insipidus
Neurogenic DI is due to mutations in the preprovasophysin gene or to destruction of either the hypothalamus (e.g., by hypothalamic tumors) or the posterior pituitary (e.g., by metastatic disease). Thus excessive water is lost in the urine. People with neurogenic diabetes insipidus have a high urine volume and a low urinary osmolality ( Table 5.1 ), accompanied by a high plasma osmolality with inappropriately low ADH levels. If fluids are withheld, these patients continue to produce an excessive urinary volume and a dilute urine. Note that the receptor for ADH ( vasopressin-2, or V2, receptor ) is intact and will respond to exogenous ADH administration. Thus ADH treatment will decrease urinary volume and increase urinary osmolality and will decrease plasma osmolality.
| Neurogenic | Nephrogenic | Psychogenic | |
|---|---|---|---|
| Plasma osmolality | ↑ | ↑ | ↓ |
| Urine osmolality | ↓ | ↓ | ↓ |
| Plasma ADH | Low | Normal to high | Low |
| Urine osmolality after mild water deprivation | No change | No change | ↑ |
| Plasma ADH after water deprivation | No change | ↑ | ↑ |
| Urine osmolality after administration of ADH | ↑ | No change | ↑ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree