Hyperthyroidism in the Neonatal Period and Childhood
Stephen H. Lafranchi
Cheryl E. Hanna
Hyperthyroidism is less common in children and adolescents than in adults, and it is rare in neonates. In all three groups, as in adults, the cause is nearly always Graves’ disease. Many aspects of hyperthyroidism (and Graves’ disease) in neonates, children, and adolescents are similar to those in adults, but a few are age specific. For example, in neonates, the hyperthyroidism is transient, but it may have effects on growth and development of the nervous system. In children, hyperthyroidism has effects on growth, pubertal development, and the skeleton that do not occur in adults. Graves’ ophthalmopathy is seldom as severe as in adults, and localized myxedema is extremely rare. Furthermore, the differential diagnosis of hyperthyroxinemia is different in childhood; hyperthyroidism is the most frequent cause, rather than pregnancy and estrogen therapy, and generalized resistance to thyroid hormones and inherited abnormalities in thyroxine (T4) transport proteins are relatively more common than in adults. With respect to treatment of Graves’ hyperthyroidism, there is more reluctance to undertake destructive therapy than in adults, although radioiodine therapy is gaining acceptance in children over 10 years of age.
Neonatal Hyperthyroidism
Incidence
Neonatal hyperthyroidism is rare, accounting for at most 1% of the cases of hyperthyroidism in childhood, and is nearly always associated with maternal Graves’ disease. Usually the mother has a history of Graves’ hyperthyroidism; in many cases, the mother had hyperthyroidism during pregnancy but sometimes she had received destructive antithyroid treatment before pregnancy and was taking T4. A few mothers have had chronic autoimmune thyroiditis (Hashimoto’s disease) rather than Graves’ disease. Neonatal Graves’ thyrotoxicosis occurs in fewer than 2% of infants born to mothers who had Graves’ thyrotoxicosis during or before pregnancy (1), although a few neonates with mild thyrotoxicosis may escape detection. The condition occurs with equal frequency in boys and girls.
Pathogenesis
Graves’ hyperthyroidism in neonates is caused by the transplacental passage of thyrotropin (TSH) receptor–stimulating antibodies (TRS-Abs) (2,3,4,5,6), meaning that the mother must be producing these antibodies late in gestation. The mothers of many affected neonates have hyperthyroidism during pregnancy, but that is not essential, as noted above. In those mothers who did have hyperthyroidism during pregnancy, the clinical severity of their thyrotoxicosis is a poor predictor of neonatal thyroid status, but measurements of TRS-Abs in maternal serum are useful in this regard (2,7,8,9,10,11). Hyperthyroidism is likely in infants of mothers who have high serum TRS-Ab values, for example, greater than 500% of control values in assays based on inhibition by serum of binding of radiolabeled TSH to TSH receptors (10,11). Assays of this type do not measure biologic activity, and the prediction of neonatal thyrotoxicosis is more accurate when TRS-Abs are measured by biologic assays (7,9) (see Chapters 13B and 18A).
Serum TRS-Abs should be measured starting at 22 weeks’ gestation in pregnant women with Graves’ hyperthyroidism to identify at-risk neonates. Measurement of TRS-Ab in the neonates also may be useful in identifying those likely to develop thyrotoxicosis if it is not already present, as in newborns of mothers who are receiving an antithyroid drug (12). The occurrence of hyperthyroidism only in neonates whose mothers have high serum TRS-Ab values indicates that transplacental passage of the antibodies is limited.
Transplacental passage of maternal TRS-Abs can cause fetal as well as neonatal hyperthyroidism. Placental transport of TRS-Abs from maternal to fetal circulation increases from 5% to 8% of maternal levels at 15 weeks’ gestation to approximately maternal levels by 30 weeks’ gestation (13). Fetuses with hyperthyroidism have tachycardia, hyperactivity, and poor growth, and skeletal and brain maturation may be accelerated; fetal hydrops has been described. Fetal goiter presenting after 22 weeks’ gestation may develop either from transplacental passage of TRS-Abs causing fetal hyperthyroidism or from fetal hypothyroidism due to transplacental passage of antithyroid medication. Fetal ultrasonography, along with an assessment of fetal skeletal maturation and heart rate, may be able to distinguish between the two causes of goiter (14). Intrauterine death may also occur, particularly if the mother has severe (and usually untreated) hyperthyroidism (13). How often fetal hyperthyroidism occurs is not known, but in mothers treated with an antithyroid drug, it is probably uncommon (see Chapter 56).
Graves’ hyperthyroidism in neonates subsides spontaneously as the maternal TRS-Abs disappear from the infant’s serum. The serum half-life of immunoglobulin G in neonates is about 14 days; depending on the serum TRS-Ab concentrations at the time of delivery, the thyrotoxicosis disappears in 3 to 12 weeks.
A rarer form of neonatal hyperthyroidism is not associated with maternal autoimmune thyroid disease and persists indefinitely (13,15,16). It is caused by germline mutations in the transmembrane region of the TSH receptor that result in constitutive activation of the receptor (see Chapter 20).
These mutations may be inherited as an autosomal-dominant trait or occur sporadically as new mutations (16,17,18,19). A recent review of 152 familial cases and 15 sporadic cases caused by germline mutations in the TSH receptor gene showed sporadic patients to present at a younger age (most in infancy) with smaller thyroid glands and often with eye findings (proptosis, exophthalmos, prominent eyes, and stare), while familial cases most often occur in older children or adults with goiter or multinodular goiter and with less common eye findings (20). Somatic gain of function mutations in the TSH receptor gene have been reported to cause a toxic adenoma and hyperthyroidism in infancy (21,22). Cases of neonatal hyperthyroidism associated with McCune–Albright syndrome also have been reported (23); these are caused by somatic activating mutations in the α-subunit of the guanine nucleotide–binding protein (G protein), which is a component of the adenylyl cyclase signal transduction pathway (24).
These mutations may be inherited as an autosomal-dominant trait or occur sporadically as new mutations (16,17,18,19). A recent review of 152 familial cases and 15 sporadic cases caused by germline mutations in the TSH receptor gene showed sporadic patients to present at a younger age (most in infancy) with smaller thyroid glands and often with eye findings (proptosis, exophthalmos, prominent eyes, and stare), while familial cases most often occur in older children or adults with goiter or multinodular goiter and with less common eye findings (20). Somatic gain of function mutations in the TSH receptor gene have been reported to cause a toxic adenoma and hyperthyroidism in infancy (21,22). Cases of neonatal hyperthyroidism associated with McCune–Albright syndrome also have been reported (23); these are caused by somatic activating mutations in the α-subunit of the guanine nucleotide–binding protein (G protein), which is a component of the adenylyl cyclase signal transduction pathway (24).
Clinical Manifestations
The birth weight of neonates with hyperthyroidism is often low. Delivery may be premature, according to postconception estimates, although skeletal maturation may be accelerated as a result of hyperthyroidism in utero (25). They may have microcephaly, enlargement of their cerebral ventricles (26), frontal bossing, or triangular facies. Hyperphagia is common, but weight gain is poor despite a high caloric intake. They are irritable and difficult to console. Fever and diarrhea may also occur. Most have prominent eyes and a small diffuse goiter; rarely, the goiter may be large enough to cause tracheal obstruction. Tachycardia and bounding pulses are almost invariably present, and cardiomegaly, congestive heart failure, cardiac arrhythmias, systemic and pulmonary hypertension, jaundice, hepatosplenomegaly, and thrombocytopenia also may occur. Premature infants with hyperthyroidism have similar abnormalities (27).
The onset, severity, and duration of symptoms in neonates with Graves’ thyrotoxicosis are variable. Some are thyrotoxic at birth, but those whose mothers were receiving an antithyroid drug at the time of delivery may not become thyrotoxic for several days, until the drug is metabolized. Rarely, antibodies that block TSH action as well as those that stimulate TSH action are passed transplacentally to the fetus; in one case, the blocking antibodies prevented the effect of the stimulating antibodies for 4 to 6 weeks, thus delaying the onset of hyperthyroidism (28).
Diagnosis and Treatment
The diagnosis of neonatal hyperthyroidism should be confirmed by measurements of serum total and free T4 and serum TSH (see Chapter 13B). Indeed, cord blood for these measurements should be obtained in any infant delivered by a woman with a history of Graves’ hyperthyroidism. It is important to note that the ranges for serum total and free T4 [and triiodothyronine (T3)] are higher in normal neonates than in normal adults (see Chapter 53). The therapy of Graves’ disease during pregnancy and its effects on the fetus are described in detail in Chapter 56.
Treatment should be initiated promptly in neonates with obvious thyrotoxicosis without waiting for laboratory confirmation. Methimazole (MMI), 0.25 to 1 mg/kg daily, should be administered orally every 8 to 12 hours to inhibit thyroid hormone synthesis. Propylthiouracil (PTU) is no longer recommended because it has more frequent and severe side effects, including a small risk of severe hepatotoxicity leading to liver failure requiring transplantation (29,30). Since children appear to be at higher risk than adults for these severe side effects, the American Thyroid Association, the American Association of Clinical Endocrinologists, and the Endocrine Society published management guidelines recommending against the use of PTU as first-line treatment of Graves’ disease in children (31,32), and the U.S. Food and Drug Administration has issued a black-box warning (33). The response to an antithyroid drug may be erratic in extremely premature infants, and they require more careful monitoring than do infants of more mature gestational age (27). In severely ill infants, a β-adrenergic antagonist such as propranolol, 2 mg/kg daily, is helpful in slowing the pulse rate and reducing hyperactivity. Inorganic iodine also may be given to inhibit thyroid hormone synthesis and release, in the form of low doses of Lugol’s solution or potassium iodide (see Chapter 32). A more experimental, but apparently effective, method for giving iodine has been to administer iopanoic acid, an iodinated cholecystographic agent, in a dose of 100 to 200 mg daily or 500 mg every third day; this iodine-rich agent inhibits thyroid secretion of T4 and T3 and the extrathyroidal conversion of T4 to T3 (it is no longer available in the United States) (34,35,36). Neonates with severe clinical hyperthyroidism are often given a glucocorticoid, which in high doses decreases extrathyroidal conversion of T4 to T3 and also may inhibit thyroid hormone secretion. Digoxin may be necessary for treatment of heart failure.
The rare neonates with hyperthyroidism resulting from a TSH receptor mutation or McCune–Albright syndrome also should be treated with MMI initially. Since their hyperthyroidism is permanent, however, they should be treated by thyroidectomy or radioiodine later (17,18,19,20,37).
Fetal hyperthyroidism should be suspected in a fetus with a heart rate exceeding 160 beats/min after 22 weeks’ gestation, hyperactivity, and poor growth. Fetal hyperthyroidism may have deleterious skeletal and neurologic effects; therefore, treatment should be considered if it is severe. Thyrotoxic fetuses may be treated by giving the mother an antithyroid drug; the dose should be adjusted to maintain a fetal heart rate of about 140 beats/min (38). Treatment should be monitored clinically and by ultrasonography; an enlarging thyroid gland is evidence of overtreatment. Normal reference values for fetal thyroid gland size throughout gestation have been established (39). Fetal blood sampling by cordocentesis can be done for laboratory tests (40,41), but can result in complications including fetal loss (13). Maternal and fetal thyrotoxicosis discovered during labor has been effectively treated with labetalol (42).
Prognosis
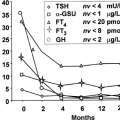
In most neonates, improvement is rapid, and treatment can be tapered and discontinued in several weeks or months, according to the results of clinical examination and measurements of serum free T4 and TSH. In rare infants in whom hyperthyroidism persists longer than 4 to 6 months, it more likely is due to a TSH receptor mutation or the McCune–Albright syndrome than Graves’ disease.
The mortality rate secondary to prematurity, congestive heart failure, and airway obstruction was estimated to be as high as 25% in the past, but it is considerably lower now. Long-term morbidity, which includes growth retardation, craniosynostosis, hyperactivity, and impairment of intellectual function, is common (15,26,43), and may occur even in neonates treated promptly and adequately, suggesting that fetal hyperthyroidism permanently affects the developing skeleton and brain. In addition, central (pituitary) hypothyroidism, which may be secondary to prenatal exposure of the hypothalamus and pituitary to high serum thyroid hormone concentrations during a critical stage of development, has been reported to follow neonatal hyperthyroidism (44,45).
Hyperthyroidism in Childhood
Most cases of hyperthyroidism in children and adolescents are caused by Graves’ disease, and the proportion caused by Graves’ disease is higher than in adults. Toxic adenoma or toxic nodular goiter, the next most common etiologies, are uncommon causes of hyperthyroidism in children. Graves’ disease may begin in infancy, but it is uncommon in children less than 5 years old (46,47,48). The incidence progressively increases throughout childhood, with a peak incidence in children 11 to 15 years of age (46,47,48). Childhood Graves’ disease is still uncommon, however, accounting for fewer than 5% of all cases. The prevalence in children approximates 0.02%. Girls are more commonly affected than boys, with a female:male ratio of 3:1 to 6:1 (46,47,48,49). The incidence of Graves’ disease in children varies on a geographic or racial/ethnic basis. In Denmark, the incidence was 0.79/100,000/year between 1982 and 1988 (50). In Hong Kong Chinese children under 15 years of age, the incidence was 3.2/100,000/year for the time period 1989 to 1993, and it was 6.5/100,000/year in 1994 to 1998 (51); the increase in incidence rate for girls was statistically significant (51).
Table 55.1 Frequency of Symptoms and Signs of Thyrotoxicosis in Children and Adolescents | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Most children with Graves’ disease have a family history of some type of autoimmune thyroid disease (48), and some have other autoimmune endocrine diseases, such as type 1 diabetes mellitus and Addison’s disease (52). In one series, 4 of 109 children with Graves’ disease previously had exhibited a picture of Hashimoto’s thyroiditis with hypothyroidism or euthyroidism from 1.5 to 2.8 years prior to the diagnosis of Graves’ disease (53). Nonendocrine autoimmune disorders, such as systemic lupus erythematosus, rheumatoid arthritis, myasthenia gravis, vitiligo, idiopathic thrombocytopenic purpura, and pernicious anemia, also have been described in these children (52). Human leukocyte antigen DR haplotypes differ in patients according to their age of onset (see Chapter 18A) (54). In addition, Graves’ hyperthyroidism is more common in children with Down syndrome [6.5 cases/1,000 patients in one registry (55)], presents at a younger age and does not show a female preponderance. (55,56). Graves’ disease may also be a part of the clinical spectrum associated with the 22q11.2 deletion (DiGeorge’s) syndrome (57).
Clinical Features
The main clinical features of hyperthyroidism in children and adolescents are similar to those in adults (46,47,48,49) (Table 55.1). Excluding weight loss and atrial fibrillation, the common symptoms and signs of hyperthyroidism are at least as frequent in children as in adults (58). Onset is often insidious, and many children have what they and their parents recall as just a few symptoms for several months or even years before the child is brought for care, even though at that time a history of many symptoms is often elicited. In children, emotional lability and hyperactivity are often attributed to behavioral or other factors, and a small goiter and prominent eyes may go unnoticed. Prepubertal children tend to have a longer duration of symptoms at presentation than pubertal children (59).
Nervousness, emotional lability, behavioral changes, and deteriorating school performance are the symptoms likely to bring the child to medical attention. These symptoms often are noted by a parent or teacher rather than by the child. Referral for evaluation of hyperactivity and possible attention deficit disorder is common. Emotional outbursts, difficulty sleeping, and mood swings may lead to referral to a child psychiatrist or counselor. Fatigue, weakness, and dyspnea may lead to a reduction in activity and cessation of athletic activities. Uncommon presentations include polydipsia (60) and headache due to benign intracranial hypertension (61).
Diffuse goiter is present in virtually all children with hyperthyroidism caused by Graves’ disease. The degree of thyroid enlargement is variable, but typically is not great. Thus, thyroid enlargement is usually not the chief complaint, and children rarely have symptoms from the enlargement. The thyroid gland is nontender, smooth, and firm, and a thyroid bruit may be heard. Thyroid nodules may be present; in a recent study of 468 patients 12 to 75 years of age, 12.8% had nodules. In six patients (1.3%), the nodule was a thyroid cancer (62).
Some ophthalmopathy is common, but severe ophthalmopathy is rare. Most children have stare and wide palpebral fissures, which are signs of thyrotoxicosis. Ophthalmic
symptoms and signs such as tearing and eyelid, periorbital, or conjunctival swelling occur in about 10% to 15% of children (63,64,65,66). In a retrospective review of 152 children with Graves’ disease followed at Children’s Hospital of Philadelphia, 26 of 152 (17%) were referred for ophthalmologic evaluation. Proptosis was noted in 38%, and mild punctuate staining in 12%. No patients were identified with strabismus or optic neuropathy (67). Another large series also found 27 of 163 (17%) children were referred to ophthalmology (68). Exophthalmos is rarely the presenting complaint (69). Eye pain, diplopia, and ophthalmoplegia are rare. The stare and wide palpebral fissures improve with treatment of hyperthyroidism; ophthalmopathy may persist but rarely requires treatment (see Chapter 18B). A survey of European endocrinologists who treat pediatric patients with Graves’ disease found a higher incidence of orbital disease in countries where teen smoking was more prevalent (70). Investigators at Texas Children’s Hospital found an association between elevated initial levels of TRS-Abs and the development of Graves’ ophthalmopathy (71).
symptoms and signs such as tearing and eyelid, periorbital, or conjunctival swelling occur in about 10% to 15% of children (63,64,65,66). In a retrospective review of 152 children with Graves’ disease followed at Children’s Hospital of Philadelphia, 26 of 152 (17%) were referred for ophthalmologic evaluation. Proptosis was noted in 38%, and mild punctuate staining in 12%. No patients were identified with strabismus or optic neuropathy (67). Another large series also found 27 of 163 (17%) children were referred to ophthalmology (68). Exophthalmos is rarely the presenting complaint (69). Eye pain, diplopia, and ophthalmoplegia are rare. The stare and wide palpebral fissures improve with treatment of hyperthyroidism; ophthalmopathy may persist but rarely requires treatment (see Chapter 18B). A survey of European endocrinologists who treat pediatric patients with Graves’ disease found a higher incidence of orbital disease in countries where teen smoking was more prevalent (70). Investigators at Texas Children’s Hospital found an association between elevated initial levels of TRS-Abs and the development of Graves’ ophthalmopathy (71).
Cardiac findings include tachycardia, a widened pulse pressure, and hyperactive precordium. Mitral valve prolapse and mitral regurgitation which improved with antithyroid drug treatment has been reported at presentation in children with Graves’ disease (72). Occasional children complain of heart “racing” or pounding. Left ventricular reserve may decrease, accounting at least in part for exercise intolerance (73), but arrhythmias and congestive heart failure almost never occur.
Most children with thyrotoxicosis have an increased appetite. Although weight loss is not as common as in adults, the children do not gain weight normally, indicating that the increase in appetite is inadequate to meet their caloric needs. BMI and weight for height have been shown to be low at presentation in both prepubertal and postpubertal boys and girls (74,75,76). On the other hand, some children truly overeat and therefore gain an excessive amount of weight. Increased stool frequency occurs in a minority of children, but diarrhea is rare. Once antithyroid treatment is instituted, some children continue to eat excessively and then may become obese. A conscious effort should be made to decrease excessive caloric intake after therapy is initiated.
Acceleration of linear growth is common in children with thyrotoxicosis, and many have an increase in height percentiles on the growth chart. The acceleration in growth is accompanied by increased epiphyseal maturation and advancement in bone age. Despite advancement in skeletal maturation, prepubertal boys and girls with Graves’ disease enter puberty at an age similar to the normal population (74,76). Studies following prepubertal and pubertal girls (74,75) and boys (76) to final height report adult height to be above target height in most patients. Thyrotoxicosis may impair mineralization of bone (77). At the time of diagnosis, bone mineral density is decreased; in one series 42% of children had severe osteopenia (78). Effective therapy of hyperthyroidism results in restoration of normal bone mass for age (78).
Most children with thyrotoxicosis have a fine tremor and brisk deep tendon reflexes. The seizure threshold may decrease, and seizures are a rare presenting feature of thyrotoxicosis (79). In children under 3 years of age, thyrotoxicosis may cause language delay or more global developmental delay, which may or may not be reversible with treatment (80). Muscle weakness and fatigue are common but seldom severe. Periodic paralysis has been described in children (81), but it is probably even rarer than in adults.
Heat intolerance and excessive perspiration occur in more than 30% of children. The skin is often warm and moist, and occasionally it is flushed. Localized myxedema and thyroid acropachy almost never occur in children with Graves’ disease.
Diagnosis
For children and adolescents who present with obvious symptoms and signs of thyrotoxicosis, diffuse goiter, and ophthalmopathy, there should be no confusion regarding the diagnosis of hyperthyroidism or its cause. In such children, laboratory tests confirm the presence of hyperthyroidism, gauge its biochemical severity, and establish a baseline for treatment.
In others, the diagnosis of hyperthyroidism may not be so obvious. These patients include seemingly asymptomatic children with diffuse goiter, painful goiter, or a thyroid nodule, and children with only a few symptoms or signs of thyrotoxicosis, such as weight loss, poor attention span and worsening school performance, or emotional lability. In these children, laboratory tests are indicated primarily to identify the presence of thyrotoxicosis. In other children, the possibility of thyrotoxicosis is considered only after thyroid function tests are done for some other reason, such as hyperactivity.
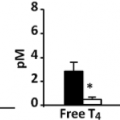
The most appropriate tests in either group are measurements of serum TSH, free T4, and T3 levels (see Chapter 13B and Chapter 31). Measurement of serum total T3 is preferable to free T3, as the normal reference range for free T3 is less validated in children. Nearly all children with thyrotoxicosis, like adults, have low or undetectable serum TSH (<0.01 mU/L) (82) and high serum free T4 and T3 concentrations. If the serum TSH value is normal (or high), the possibility of resistance to thyroid hormone should be considered (see Chapter 58), and a TSH-secreting pituitary adenoma must be excluded (see Chapter 19).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree