References
No. cases
Prevalence (%)
Measurement
Criteria for hypertension (mmHg)
Nabarro [123]
256
30.5
Clinical
Not known
Rodrigues et al. [24]
34
26
Clinical
160/95
Kraatz et al. [23]
158
31.6
Clinical
160/95
Kraatz et al. [23] recalculated
50.6
140/90
Molitch [124]
639
23
Clinical
Not known
Ezzat et al. [64]
500
51
Clinical
140/90
Ohtsuka et al. [25]
64
37.5
Clinical
160/95
Ohtsuka et al. [25] recalculated
57.8
140/90
Lopez-Velasco et al. [125]
39
33.3
Clinical
140/90
Minniti et al. [17]
40
42.5
Clinical
140/90
Minniti et al. [17]
40
17.5
ABPM
136/84
Terzolo et al. [21]
16
31.2
ABPM
136/84
130
35.4
Clinical
DBP 90
Fukuda et al. [126]
65
38
Clinical
Not known
Jaffrain-Rea et al. [18]
68
51.5
Clinical
140/90
68
47
ABPM
135/85
Otsuki et al. [127]
21
57
Clinical
140/90
Pietrobelli et al. [19]
25
56
Clinical
140/90
25
40
ABPM
136/84
Weiss et al. [128]
55
38.2
Clinical
Not known
Jaffrain-Rai et al. [20]
31
45.1
Clinical
140/90
31
35.5
ABPM
136/84
Vitale et al. [14]
200
46
Clinical
140/90
Damjanovic et al. [41]
23
39
Clinical
Not known
Mestron et al. [129]
1,036
39.1
Medical records
Medical records
Colao et al. [26] (elderly pts)
57
82
Clinical
140/90
89
57.3
Clinical
140/90
Sucunza et al. [27]
60
37.7
Clinical
Not known
Ciresi et al. [15]
12
66.7
Clinical
140/90
Melnichenko et al. [31]
232
80.2
Clinical
Not known
Colao et al. [29]
45
46.7
Clinical
90 DBP
Some authors have argued that the method of BP measurement plays a key role in the divergent estimates. Minniti and colleagues [17] compared the prevalence of hypertension as diagnosed with a manual sphygmomanometer, following an accepted protocol (42.5%), with results from ambulatory BP measurement (ABPM, 17.5%) taken at regular intervals in acromegalic subjects. They found that only 40% of patients found to be hypertensive by clinical assessment were confirmed as hypertensive with ABPM. They then questioned whether hypertension in acromegaly had been over-estimated in previous studies. Subsequent studies, however, that made measurements using both clinical and ABPM techniques have reported both less discrepancy between the techniques and a higher prevalence of hypertension using ABPM [18–21]. In addition to this, the difference that Minniti et al. found may represent mainly the “white coat” hypertension phenomenon that has been found by others to be associated with the development of frank hypertension [22]. Furthermore, the evidence that hypertension is an independent and strong predictor of mortality in acromegalic patients [11, 12] was formulated from clinical assessment of BP, so the prevalences calculated using similar methods are likely to hold significance. Estimates of hypertension’s prevalence remain diverse regardless of method used to assess BP. This suggests other factors are contributing to the results’ heterogeneity.
One other possible cause of the variability in results is the use of different reference ranges and definitions for hypertension. Most of the papers that provide a prevalence of hypertension used a criterion of >140 mmHg systolic and/or >90 mmHg diastolic BP. Some papers did not report their criteria, although in papers published since 2000 it may be fair to assume that a similar definition was used. Some used only a diastolic BP >90 mmHg as a criterion and some older papers used the prior threshold of >160 mmHg systolic over >95 mmHg diastolic [23–25]. By today’s standards this would underestimate prevalence. Two of these papers define BP >140/90 mmHg but <160/95 mmHg as “borderline hypertension,” and patients in this category would have been classified as hypertensive in the other studies. If these patients are added to the prevalence figures, the prevalence of 31.6% in Kraatz and colleagues’ 1990 paper [23] becomes 50.6%. In Ohtsuka et al.’s study [25], the prevalence of 37.5% becomes 57.8%. Although this reduces the range of the results somewhat, it is still substantial. This residual variability may be due to the relatively small samples size in most studies, a result of the relative infrequency of acromegaly. Lastly, given the many factors that predispose to hypertension, it is likely that the different study populations had differing mixes of family history, race, diet, or other variables. This is particularly relevant in studies where control groups from the same population were not used.
Prevalence Compared to Control Populations
Some of the studies have included internal comparison groups, which allow a better estimation of the association between acromegaly and hypertension. One group compared the frequency of hypertension between a retrospective series of 158 acromegalic subjects and a large, local epidemiological study [23], only finding a raised prevalence of hypertension in female patients. Hypertension was found in 19.6% of acromegalics. However, when the figures were recalculated using modern definitions, hypertension is considerably more common in both sexes, with 50.6% of patients being hypertensive vs. 32.9% in the comparison population. A Japanese group found that the prevalence of hypertension was higher in a retrospective cohort of 64 acromegalics (37.5%) than in the local population (20–25%) [25]. They found that 75% of patients with acromegaly classified as hypertensive (using the old definition) had a family history of hypertension compared to 25.9% of normotensive acromegalics. This effect may have been diluted when hypertension is defined by modern standards as only 23.1% of their “borderline hypertensive” acromegalics had a positive family history. However, since a family history of hypertension increases the risk of hypertension in non-acromegalics as well, this may be a confounder rather than a specific effect.
A larger subsequent study used 200 cases and controls matched for age, sex, body mass index, and lifestyle factors and found hypertension in 46% of acromegalic cases and 25% (p < 0.0001) of controls [14]; cases had significantly higher diastolic and systolic BPs. In contrast to some other studies, gender did not influence the acromegaly-hypertension association; they also found that a family history of hypertension was more common in controls than patients, in contrast with Kraatz et al. [23]. Hypertension was positively correlated with age in both groups, although it occurred 10 years earlier in cases than in controls. The strength of this study lies in its relatively large size and the use of controls matched for factors that could otherwise confound the results (the prevalence also agrees with worldwide estimates [16]); however, there is no mention of blinding of investigators which may be a weakness.
One study looked at hypertension in a cohort of 57 patients who were diagnosed with acromegaly when older than 60, comparing them with gender and age-matched controls [26]. They found that 82% of the patients, whereas only 67% of controls were hypertensive. Mean systolic and diastolic BPs were significantly higher in cases. The authors note that many of the acromegalic patients are being treated unsuccessfully for hypertension. This may imply that hypertension associated with acromegaly is relatively refractory to treatment.
Two studies have compared controls with a mix of acromegalic patients—treatment naïve, and those receiving or who recently received unsuccessful treatment of their acromegaly. Sucunza et al. found that 37.7% of acromegalic patients (n = 19) were hypertensive compared with only 15% of controls (n = 105) [27]. Ciresi’s group also did a small study of acromegaly patients mostly receiving therapy, 12 of whom were not responding completely. They compared the 12 with active acromegaly against 21 controls and found hypertension in 66.7% of cases and 0% of controls [15]. These papers do corroborate the evidence from other stronger studies; however, they are both small and contained many patients receiving acromegaly treatment, a potentially confounding attribute as a search of the literature shows that somatostatin analogues (SSA), a mainstay of medical therapy for acromegaly, may themselves increase BP [28].
Thus, despite the wide range of prevalence findings, the association between acromegaly and hypertension appears to have a strong base of evidence. The best evidence comes from the Naples group’s case–control study of 400 subjects [14]; their result that 46% of patients with acromegaly are hypertensive by clinical assessment (compared with 25% of controls) also falls around the mean of the other studies’ results. This does not, however, provide evidence of a causal relationship.
Resolution of Hypertension with Treatment of Acromegaly?
One observation that would provide a stronger argument for causality is if treatment of acromegaly reduces or resolves hypertension. The evidence surrounding this is mixed, with some studies finding a reduction in BP while others showing no effect. There may also be differences between the different methods of treatment—medical vs. surgical.
The best study to address this question may be that of Colao et al. from 2008 [13]. They retrospectively selected 105 treatment-naïve acromegalic patients, all receiving therapy for hypertension. Hypertension was successfully controlled in only 14% of patients; the remainder continue to be hypertensive despite therapy. Subjects received a variety of treatments, which normalized GH and IGF-I levels in 72%. In this group both systolic and diastolic mean BP fell by 13.1% and 11.4% respectively (p < 0.0001), independent of treatment method. The reduction in BP was not statistically significant in patients whose acromegaly therapy was unsuccessful. This study of hypertensives found that basal systolic and diastolic BPs were correlated with GH and IGF-I levels. Furthermore, there was a significant correlation between the percentage reduction in GH/IGF-I levels and the percentage reduction in systolic and diastolic BPs.
Their findings of both a degree of dose–response relationship between GH/IGF-I levels and BP, and a significant reduction in BP and uncontrolled hypertension when acromegaly is controlled represent the strongest evidence yet that acromegaly does cause hypertension. Limitations of the study include the exclusion of hypertensive patients who were not receiving antihypertensives and the lack of blinding. These may have biased results, however, the overwhelming strengths of this study designed—unlike others—specifically to detect a change in BP with acromegaly treatment lend considerable weight to the argument that acromegaly causes a potentially reversible hypertension. The subsequent year the same group produced a study looking at patients (some of whom have also been included in the previous study after shorter follow-up) who received only SSA treatment for 5 years (excluding patients who were initially or subsequently managed surgically) [29]. They observed a reduction in hypertension greater than 50% (p = 0.027). This reduction retained significance when patients were analyzed by sex or type of SSA.
One study of 31 patients (11 hypertensives) who underwent successful surgery for acromegaly measured pre- and post-operative BP clinically and using ABPM. They found significant reductions in systolic and diastolic BPs by clinical measurement [20]. With ABPM they found that 24 h, daytime and nighttime systolic pressures fell significantly; however, corresponding diastolic changes were nonsignificant.
These papers provide focused data about whether treatment of acromegaly improves the associated hypertension by removing confounding diluent effects in other papers of small initial numbers of hypertensive patients and patients who do not respond adequately to treatment. Another paper by Delaroudis and colleagues [30] found a significant reduction in BP in 18 patients on SSAs that achieved only partial control of their GH/IGF-I levels. A Russian group recently presented data on a sample of 232 acromegalic patients, 80.2% of whom were hypertensive. They report a normalization of BP in patients whose acromegaly began before age 45, but not in others [31]. Other groups have found a link between age and cardiovascular comorbidities in acromegaly. A phenomenon also recognized in acromegalic cardiomyopathy [32], duration of disease, is likely to play a role in this. A Dutch study looking at comorbidities of patients in long-term remission from acromegaly found that compared to epidemiological data, only patients aged 50–70 were significantly more hypertensive than controls [33]. Some groups looking at whether remission of acromegaly results in resolution of hypertension have found less or no association. It should be mentioned that few of these studies focus on hypertension as a primary outcome; most are looking at changes in other conditions, usually cardiomyopathy.
Colao et al. published another study in 2008 looking at improvement in cardiovascular morbidity by different successful acromegaly treatments: surgery vs. SSAs [34]. In this case only diastolic BP decreased significantly, but with both treatment approaches. Overall, there was a nonsignificant trend to a reduction in the number of hypertensives with both treatments. This may not have reached statistical significance because the analysis subdivided hypertension into stages 1 and 2 and patients were divided by treatment, meaning small samples. A significant reduction in diastolic BP alone was also found in another paper when GH/IGF-I levels were normalized [35]. Another study found that patients in remission after surgical treatment of acromegaly had significantly lower diastolic BP than those whose surgery had failed, but hypertension prevalence and systolic BP were not significantly different [36].
A 2007 meta-analysis of 18 papers (none were randomized control trials) which examined the effect of SSAs on the heart in acromegaly found no significant effect on hypertension [37]. However, hypertension was not one of the inclusion criteria, meaning certain papers were ignored including more recent studies that used the best designs for detecting changes in hypertension. Another reason that the meta-analysis may have failed to detect a significant difference is that many of the studies were done before the currently accepted definition of remission of acromegaly. The mean level of post-treatment GH after an oral glucose tolerance test (OGTT) was 7.6 μg/L—today an accepted level for remission is <1 μg/L [38]. This incomplete remission of acromegaly may have meant that changes in BP were insignificant; however, another group have found significant changes with partial control of acromegaly (although mean nadir GH was lower at 2.35 μg/L) [30]. Other potential explanations include that the possibility that follow-up was not sufficiently long to see resolution of hypertension, possible effects of different age demographics, that heterogeneity influenced results or that low numbers of hypertensives meant that mean BP changes were small when diluted with non-hypertensive patients.
Apart from this meta-analysis, papers from other groups found no significant reduction in BP or hypertension. One series including 15 patients (4 hypertensives) [39] found no significant reduction. Two reasonably sized (43 and 61 patient) studies compared those with successful remission of acromegaly with those with partial remission and with controls; neither found any difference in BP or hypertension [40, 41]. One study of 10 patients treated with a subcutaneous SSA (octreotide) found a significant increase in mean 24 h and nighttime systolic and diastolic BPs with ABPM [28]. They hypothesize that this may be due either to vasoconstrictive effects of octreotide on the splanchnic vasculature, increasing systemic circulating volume, or because ABPM provides a better assessment of hypertension than clinical measurements. It seems possible that SSAs produce this effect only transiently, and if other studies measure BP clinically at another time to treatment this effect might be missed. Perhaps only ABPM, with its long-term measurements would detect this change.
The combination of these findings presents a mixed picture. Methodologically, the strongest studies do find a reduction in BP. However, we cannot ignore evidence from the meta-analysis and other studies that found no change. Despite having flaws in their design for detecting change in BP (most not having been designed with this aim) they demonstrate that BP reduction with treatment of acromegaly may only happen in certain patients or settings, with certain treatments, or that changes are small. More studies are required, including the use of ABPM and larger cohorts looking for changes in BP in both hypertensives and normotensives. Samples need to be large so that data can be analyzed for those whose treatment is successful and for those who remain acromegalic.
The Nature of Acromegaly-Associated Hypertension
A very small number of groups have attempted to elucidate the nature of the BP change that occurs in acromegaly. As seen above some have found that hypertension is predominantly diastolic or systolic, however there is also evidence from studies using ABPM about BP patterns in acromegaly, particularly loss of the normal circadian fall in nocturnal BP or “non-dipping.” Non-dipping has been found to be independently associated with significant cardiovascular morbidity [42]; two population studies have estimated its prevalence in a cohort of Swedish men (6.2%) [43] and in normotensive non-diabetic Finnish subjects (18.8%) [44]. Jaffrain-Rai and colleagues found that 44.1% of 68 acromegalic patients were non-dippers [18]. They analyzed to look for interaction with the glucose tolerance of patients—since diabetes mellitus is independently associated with non-dipping—but no such interaction existed. They do highlight the important fact that lack of nocturnal fall in BP may be confounded by the coexistence of sleep apnea—this could cause nocturnal hypertension and is found in acromegaly. Another study (n = 25) found a high prevalence of non-dipping in both normotensive patients (40%) and hypertensive patients with acromegaly (60%), and that non-dipping was associated with higher GH levels [19]. These agreed with a previous smaller paper [21] but contradict findings of Minniti et al. who found only one of seven hypertensive acromegalic patients were non-dippers [17]. The latter paper also found a very large difference between the prevalence of hypertension when using clinical measurements or ABPM (as mentioned above) so the population may have been atypical. Another group have presented data showing similarly high levels of non-dipping (57%) in acromegalics regardless of whether hypertensive or not [45]. The only evidence where controls were used is from a small study that found non-dipping was more common in acromegalics (62%) vs. controls (12%, p < 0.01) [21]. In summary, it appears that the non-dipping profile may be associated with acromegaly, beyond that expected for the hypertension and impaired glucose tolerance seen in the condition. Whether this effect is mediated by concomitant obstructive sleep apnea remains to be clarified.
Conclusions
Evidence from case series and case–control studies suggest strongly that acromegaly is associated with elevated BP. The prevalence remains unclear, with estimates ranging from 17.5% to over 80%, however most studies, particularly larger ones designed to look at hypertension in acromegaly find it to be between approximately 40 and 60%, and higher than in matched controls. Acromegaly may also cause the non-dipping nocturnal BP pattern.
As mentioned above, hypertension is of significance in acromegaly because of its negative impact on acromegalic cardiomyopathy and as a predictor of mortality. It appears that treatment of acromegaly can lead to an improvement of hypertension; however, there is not sufficient evidence about effects of management of hypertension on mortality in acromegaly. It also remains to be clarified whether the effects are directly from GH/IGF-I excess or indirectly e.g. from obstructive sleep apnea; there does not seem to be a consistent correlation between either GH/IGF-I levels or duration of disease and degree of hypertension [20], pointing to a complex association.
Possible Mechanisms for Hypertension in Acromegaly
Expansion of Plasma Volume
There is quite extensive evidence to suggest that acromegaly is associated with an augmentation of the body’s fluid volumes. In a cross-sectional study, one group found that average extracellular water was 6 L greater for 16 acromegalic patients than for 18 controls (p < 0.01) [46], these results were in agreement with findings from a larger population [47]. There is also a good body of evidence that these expansions in body water resolve with both surgical and medical treatment, reviewed in detail elsewhere [48, 49].
Plasma volume and extracellular water are closely related to the body’s exchangeable pool of sodium. The finding that this pool of sodium is increased in acromegalics [47, 50], including in hypertensive patients [25] lends support to this theory. The cause of this change in plasma volume and exchangeable sodium pool is not clear; however the association is consistent, so it is likely that this is at least one of the pro-hypertensive changes in acromegaly.
Direct Effect by GH or IGF-I
It has been suggested that hypertension and this expansion of plasma volume in acromegaly may also be induced indirectly via a number of pathways, including direct actions of GH and/or IGF-I on the kidney. Indeed, both GH and IGF-I receptors are expressed in the kidney [51] which has led to investigation of this as a possible link.
Direct action of GH was first suggested as early as the 1960s [52]. The majority of subsequent evidence comes mainly from animal models, non-acromegalic subjects, and from effects on channels outside the kidney as a proxy for renal activity (comprehensively reviewed elsewhere [48]). A number of studies have looked at activity of the sodium-potassium pump (Na+/K+ATPase) in other tissues, with contradictory conclusions about it being up-regulated [53] or down-regulated [54] in acromegaly. Given this inconsistency in the literature, the paucity of human trials and difficulty in distinguishing activity attributable to GH or IGF-I we cannot be sure of a GH-mediated effect.
GH can cause an increase in the glomerular filtration rate (GFR) of normal subjects [55] and a raised GFR is seen in acromegalic patients [50, 56]. However, this is also seen with administration of IGF-I, which suppresses GH secretion, in normal subjects; so IGF-I may be the direct mediator [57]. The direction of causality between increased plasma volume and increased GFR also remains to be defined.
Renin-Angiotensin-Aldosterone System and Brain Natriuretic Peptide
The renin-angiotensin-aldosterone (RAA) system and brain natriuretic peptide (BNP) have both been implicated as possible indirect mechanisms of hypertension in acromegaly. However, some of the results have subsequently been found to be irreproducible. One group produced a paper thoroughly examining this integrated endocrine axis [58]. They found that there was no significant difference between active and remitted acromegalic subjects for plasma renin activity, aldosterone levels, plasma atrial natriuretic peptide (ANP) or amount of natriuresis, nor was there a significant change in BNP levels after treatment of acromegalic subjects. Limitations of the study include the fact that none of the patients had significant hypertension (all <160/90 mmHg) so we cannot be sure these changes do not occur in hypertensives, although plasma volume was significantly increased in active patients. Also, their definition of remission was less strict than today’s so it is possible that treatment of acromegaly was incomplete. Furthermore, some have criticized the accuracy of the early ANP/BNP assays [52, 59]. This paper does, however, provide evidence that BNP and the RAA system may not be the key mediators of acromegaly-associated hypertension, which is supported by other groups’ findings concerning the RAA system [23, 60, 61]. Many do, however, believe it is implicated somehow in acromegaly [62] and since it seems to be important in BP changes when GH is given to controls [63], further investigation is warranted.
Insulin-Mediated Mechanisms
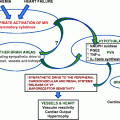
A common feature of acromegaly is insulin resistance and resultant hyperinsulinemia [64]. Some have proposed that insulin causes hypertension through a number of mechanisms, as outlined in Fig. 8.1. Many groups have found that hypertension is more prevalent among acromegalic patients with impaired glucose tolerance or diabetes than those without [10, 18, 65, 66], although others’ findings disagree [64] and some found the association only between abnormalities of carbohydrate metabolism and non-dipping profiles [19]. It is also not clear whether there is confounding; impaired carbohydrate metabolism and hypertension may commonly coexist, because they are both found in severe acromegaly or certain patient subgroups. However, one group found no interaction between hypertension or carbohydrate metabolism and either GH or IGF-I levels [18].
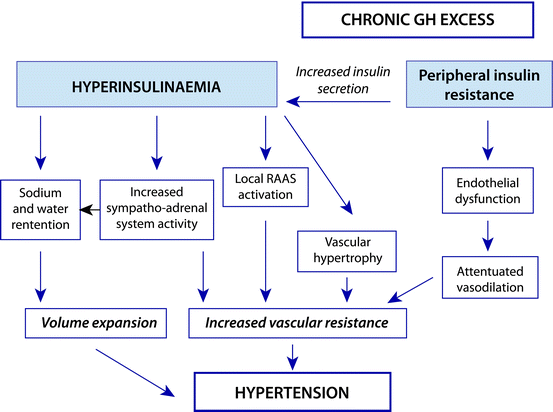

Fig. 8.1
Summary of proposed mechanisms of insulin-mediated hypertension in acromegaly. Reprinted with permission: Bondanelli et al. [76]
Evidence from non-acromegalic patients does provide a number of putative links. Insulin is known to have its own role in sodium homeostasis, causing increased tubular reabsorption [67], and is thought to cause an increase in sympathetic nervous system activity, activation of the RAA, and vascular effects [67, 68]. There is, however, a dearth of evidence from acromegalic patients and we are therefore unsure of the role of insulin in this association.
Sympathetic Activity
Increased sympathetic tone in acromegaly has been suggested as the cause of hypertension and while some have found raised plasma catecholamines in active acromegaly vs. controls [69, 70] (possibly because acromegalic patients were older than controls in Rozenberg et al. [69]), most have found urinary and plasma levels to be normal [71–74]. Bondanelli and colleagues found normal plasma catecholamine levels, but an absence of their normal nocturnal drop in acromegalic patients, which returned after surgical treatment of acromegaly [75]. This may have relevance to the non-dipping BP profile, but may also be related to obstructive sleep apnea. Once again, there is insufficient evidence to convincingly confirm or refute sympatho-adrenomedullary hyperactivity as being of major importance in acromegaly-associated hypertension. The issue is further confused by the fact that insulin resistance, sleep apnea, and expansion of plasma volume may all cause abnormal autonomic outflow [76]. Increased sympathetic activity may, therefore, be a side product of these conditions, the intermediate link between them and hypertension, or completely unrelated.
Cardiovascular Effects
Patients with acromegaly have been observed to have a hyperkinetic heart with increased output and cardiac index [77, 78]. Coupled with evidence of dysfunctional regulation of systemic vascular resistance, the possibility of this as a cause of hypertension in acromegaly has been raised. Although systemic vascular resistance is thought to be reduced in uncomplicated acromegaly, it appears that cardiac output may be distributed abnormally peripherally as measurements of brachial artery hemodynamics revealed increased local resistance compared with controls [79]. Others have found impaired endothelium-dependent vasodilation in the upper limb that may explain this [80]. Endothelium-dependent structural changes in vessels have also been reported, with increased intima-media thickness (IMT) in active acromegalics compared with healthy controls and inactive patients [81], although when patients were matched with controls for vascular risk factors there was no significant difference [82]. Increased IMT can be a result of hypertension, impaired glucose tolerance, and other cardiovascular risk factors [77]. Increased IMT can also be a primary change to cause hypertension [83]. The discovery of increased IMT in normotensive acromegalic patients [84] suggests that this change may contribute to hypertension; however it is unclear whether this is a direct effect of GH/IGF-I or an indirect effect of impaired glucose tolerance. It appears that vascular dysfunction and structural change may be present in acromegaly and may contribute to hypertension; however, further studies are required to clarify this.
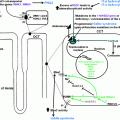
Sleep Apnea Syndrome
As mentioned previously, one possible intermediate link between acromegaly and hypertension is sleep apnea syndrome (SAS). SAS is seen commonly in acromegaly—estimates range from 20 to 80% [85]—and hypertension is associated with SAS in 40–60% in the general population [86]. Despite this, there is little recognition of this in the literature concerning acromegaly and hypertension and very few papers investigate SAS as a potential confounding comorbidity. One study mentions that the severity of SAS was greater in hypertensive acromegalics than normotensives [87]. The proposed pathophysiology of hypertension in SAS is shown in Fig. 8.2, and includes alterations in sympathetic activity and endothelial dysfunction as discussed above. It also includes reduced nitric oxide (NO) production, which has been detected by one group [88]. SAS of course is not limited to acromegalics; in non-acromegalic patients with SAS, GH secretion and IGF-I levels have been reported to be reduced, perhaps because of elevated cortisol due to the hypoxemia and resultant frequent near-awakening (for review see [89]).
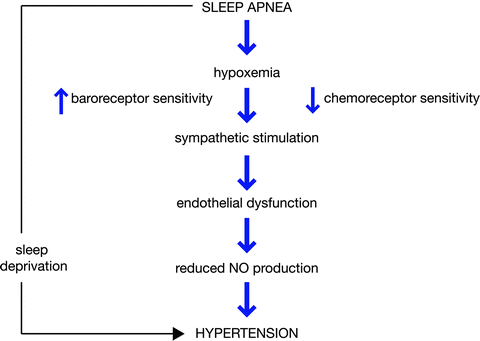

Fig. 8.2
Proposed mechanism of development of hypertension in sleep apnea. Reprinted with permission: Fatti et al. [86]
Conclusion: Acromegaly
These represent some of the major mechanistic theories concerning the association between acromegaly and hypertension. Hypertension is probably, as in the general population, a product of numerous factors in acromegaly. The evidence reviewed above shows some of these possible factors individually and the overlap between them. Future research must consider these several issues in an integrated fashion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree