Hydroxyurea therapy offers promise for ameliorating the clinical course of children with sickle cell disease (SCD). Hydroxyurea is a prototypic therapeutic option; it can be administered with minimal side effects, has a relatively wide therapeutic window, and has mechanisms of action that address pathophysiologic pathways of sickling, vaso-occlusion, hemolysis, and organ damage. There are limited data regarding hydroxyurea’s ability to prevent or diminish organ dysfunction, and the long-term risks of hydroxyurea therapy remain incompletely defined. Although clinical trials are underway to address long-term issues, hydroxyurea remains an effective but underutilized therapy for SCD.
Sickle cell disease (SCD) refers to a group of genetic hemolytic anemias in which the erythrocytes have a predominance of sickle hemoglobin (HbS) due to inheritance of a β-globin mutation (β S ). The β S mutation is the result of a single amino acid substitution (HbS, HBB Glu6Val ) in the β-globin of the hemoglobin heterotetramer, thus forming HbS. Affected individuals typically are homozygous for the sickle mutation (HbSS) or have a compound heterozygous state (eg, HbSC, HbS β-thalassemia). The β S mutation creates a hydrophobic region that, in the deoxygenated state, facilitates a noncovalent polymerization of HbS molecules that damages the erythrocyte membrane and changes the rheology of the erythrocyte in circulation, causing hemolytic anemia, vaso-occlusion, and vascular endothelial dysfunction.
SCD is the most common inherited hemolytic anemia in the United States. Approximately 70,000 to 100,000 individuals in the United States are affected, most commonly those who have ancestry from Africa, the Indian subcontinent, the Arabian Peninsula, or the Mediterranean Basin. Worldwide, millions of persons are affected with SCD, especially in regions with endemic malaria, such as Africa, the Middle East, and India. SCD is characterized by a lifelong hemolytic anemia with an ongoing risk for acute medical complications and inexorable accrual of organ damage in most affected individuals.
There is wide variability in the phenotypic severity of SCD that is not well understood. This variation can be explained partly by differences in the total hemoglobin concentration, the mean corpuscular hemoglobin concentration, erythrocyte rheology, the percentage of adhesive cells, the proportion of dense cells, the presence or absence of α-thalassemia, and the β-globin haplotype. The percentage of fetal hemoglobin (HbF), however, is perhaps the most important laboratory parameter influencing clinical severity in SCD. In unaffected individuals, HbF comprises only 5% of the total hemoglobin by age 3 to 6 months and falls to below 1% in adults. In contrast, patients with SCD typically have HbF levels ranging from 1% to 20% and those with genetic mutations leading to hereditary persistence of HbF (HPFH) can have HbF levels that reach 30% to 40% of the total hemoglobin.
Based on the observation that infants with SCD have few complications early in life, it was hypothesized that HbF, the predominant hemoglobin in fetal and infant stages of life, might ameliorate the phenotypic expression of SCD. In addition, compound heterozygotes for the sickle mutation and HPFH are relatively protected from severe clinical symptoms. Subsequently, it was shown that increased HbF percentage is associated with decreased clinical severity in SCD, using endpoints, such as the number of vaso-occlusive painful events, transfusions, and hospitalizations. HbF does not, however, seem to protect from some complications, perhaps because the HbF levels were inadequate to provide protection. A potential threshold of 20% HbF is suggested, above which patients experience fewer clinical events. The % HbF also has emerged as the most important predictor of early mortality in patients with SCD.
Although the genetic and molecular pathophysiology of SCD are well described and understood in considerable detail, there has been disappointing progress toward definitive, curative therapy. Bone marrow transplantation offers a cure but currently requires an HLA-matched sibling donor for best results. This requirement limits the number of patients who can benefit from this approach. Moreover, even using a matched sibling donor, bone marrow transplantation remains associated with considerable morbidity (primarily graft-versus-host disease) and low, but not negligible, mortality.
In lieu of curative therapy, one approach given considerable effort over the past 25 years has been the pharmacologic induction of HbF beyond the fetal and newborn period. Several pharmacologic agents have shown promise, including demethylating agents, such as 5-azacytidine and decitabine, and short-chain fatty acids, such as butyrate, but each has limitations in route of administration, safety, or sustained efficacy. Hydroxyurea, in contrast, has a long and growing track record in inducing HbF in patients with SCD. In addition, hydroxyurea has a variety of salutary effects on other aspects of the pathophysiology of SCD, such as increased erythrocyte hydration, improved rheology, and reduced adhesiveness. Hydroxyurea also decreases leukocyte count, and releases nitric oxide. This article reviews the usefulness of hydroxyurea for children with SCD but is not intended to be an exhaustive review of the drug’s biochemistry, its therapeutic rationale, or previously published data. Interested readers may read more thorough reviews of hydroxyurea for the management of SCD. This article is intended as a practical user’s guide for clinicians who wish to know how and why treatment with hydroxyurea should be considered for children with SCD.
An ideal drug for sickle cell disease?
Hydroxyurea may be an ideal therapeutic agent for use in children with SCD. It has excellent bioavailability after oral administration; requires only once-daily dosing, which improves medication adherence; has few if any immediate side effects; has predictable hematologic toxicities that are dose dependent, transient, and reversible; and has potential benefits against multiple pathophysiologic mechanisms of SCD. Although several therapeutic agents currently under development address specific aspects of the pathophysiology of SCD, only hydroxyurea offers a broad range of beneficial effects that collectively can ameliorate the overall clinical severity of disease.
The drug is classified as an antimetabolite and antineoplastic agent. The exact mechanism of its antineoplastic activity is not elucidated fully but believed to be S-phase specific. Hydroxyurea is converted in vivo to a free radical nitroxide that quenches the tyrosyl free radical at the active site of the M2 subunit of ribonucleotide reductase. As a potent ribonucleotide reductase inhibitor, hydroxyurea blocks the conversion of ribonucleotides to deoxyribonucleotides, which interferes with the synthesis of DNA without any effects on RNA or protein synthesis. The drug is used widely in oral doses (ranging from 20 to 80 mg/kg/d) for the long-term treatment of chronic myeloproliferative disorders, such as polycythemia vera and essential thrombocythemia. In combination with reverse transcriptase inhibitors (eg, didanosine), hydroxyurea is finding a role within HIV therapy as a virostatic agent that produces potent and sustained viral suppression.
In patients with hemoglobinopathies, the myelosuppressive and cytotoxic effects of hydroxyurea seem to induce erythroid regeneration and the premature commitment of erythroid precursors, with resulting increased production of HbF-containing reticulocytes and total HbF. Additional pharmacologic effects of hydroxyurea that may contribute to its beneficial effects in SCD include increasing erythrocyte HbF through nitric oxide dependent pathways, decreasing the neutrophil count, increasing erythrocyte volume and hydration, increasing deformability of sickle erythrocytes, and altering the adhesion of sickle erythrocytes to the endothelium. The release of nitric oxide directly from the hydroxyurea molecule should allow beneficial local effects on the endothelium, thereby ameliorating the vaso-occlusive process and limiting vascular dysfunction.
Clinical experience
Preclinical studies in anemic cynomolgus monkeys showed that hydroxyurea increased HbF levels. Pilot trials in patients with SCD demonstrated that hydroxyurea also increased HbF in humans and caused little short-term toxicity. These proof-of-principle experiments were critical first steps toward an important multicenter phase I/II trial involving adults with HbSS, which identified the short-term efficacy and toxicities of hydroxyurea used at maximum tolerated dose (MTD).
Developed on the basis of favorable results from the phase I/II trial, the National Heart, Lung, and Blood Institute (NHLBI) sponsored the pivotal Multicenter Study of Hydroxyurea (MSH), a double-blinded, placebo-controlled, randomized control trial conducted from 1992 to 1995 in 21 centers in the United States. and Canada. Two hundred and ninety-nine adult patients with HbSS were randomized (152 on hydroxyurea and 147 received placebo) but because of the beneficial effects observed, the trial was stopped early and only 134 subjects completed the planned 24 months of treatment. The hydroxyurea-treated subjects had a 44% reduction in painful crises per year (2.5 events per year versus 4.5 events per year) and a 58% reduction in median annual hospitalization rate for painful crisis (1.0 versus 2.4). In addition there were significantly fewer hydroxyurea-treated subjects who developed acute chest syndrome (ACS) (25 versus 51) and who received blood transfusions (48 versus 73); the number of units of blood transfused also was significantly less (336 versus 586). The incidence of death and stroke did not differ between the two treatment arms; there were no deaths related to hydroxyurea treatment and none of the patients who had received hydroxyurea developed cancer during the trial. The study did not address long-term safety or potential reversibility or prevention of chronic organ damage.
The results of this study led the Food and Drug Administration in 1998 to add to the indications for hydroxyurea, “to reduce the frequency of painful crises and to reduce the need for blood transfusions in adult patients with sickle cell anemia with recurrent moderate to severe painful crises.” This additional labeling refers only to adults severely affected by painful events rather than the broader spectrum of patients with SCD. Now, 10 years later, there is no change in the manufacturer’s drug labeling for hydroxyurea. Therefore, at this time, all children or patients with mild-to-moderate disease severity or those who do not have painful events but who have ACS or end-organ damage require off-label usage.
The initial success of hydroxyurea in adults led to the first pediatric multicenter phase I/II trial, known as HUG-KIDS, from 1994 to 1996. Eighty-four children ages 5 to 15 years with severe HbSS disease (defined as three or more painful events within the year before entry, three episodes of ACS within 2 years of entry, or three episodes of ACS or pain within 1 year of entry) were enrolled. Sixty-eight reached MTD and 52 were treated at MTD for 12 months. Similar hematologic effects were seen as in the MSH trial with decreased hemolysis (increased Hb and decreased reticulocytosis, decreased lactate dehydrogenase, and decreased total bilirubin), macrocytosis, improved erythrocyte hydration, myelosuppression, and increased HbF and F cells ( Table 1 ). Laboratory toxicities were mild and reversible with temporary interruption of the medication, and no life-threatening clinical adverse events were observed. Subsequent evaluation of this cohort revealed no adverse effect on height or weight gain or pubertal development. Predictors of HbF response were complex, but a higher treatment HbF was associated with higher baseline HbF, Hb, white blood cell count (WBC), and reticulocytes and compliance.
| Adults | Children | |
|---|---|---|
| MTD (mg/kg/d) | 21.3 | 25.6 |
| Δ Hb (g/dL) | +1.2 | +1.2 |
| Δ MCV (fL) | +23 | +14 |
| Δ HbF (%) | +11.2 | +9.6 |
| Δ Reticulocytes (10 9 /L) | −158 | −146 |
| Δ WBC (10 9 /L) | −5.0 | −4.2 |
| Δ ANC (10 9 /L) | −2.8 | −2.2 |
| Δ Bilirubin (mg/dL) | −2.0 | −1.0 |
Short-term clinical efficacy in children initially was reported in small open-label studies. In a small, randomized study from Belgium, children with HbSS treated with hydroxyurea had significantly fewer hospitalizations for pain, with shorter lengths of stay, compared with those receiving placebo. Additional European data showed improved laboratory and clinical response without significant toxicity and no growth or pubertal delay. Follow-up studies have revealed continued efficacy in association with long-term hydroxyurea use in children, including a sustained HbF response greater than 20% using hydroxyurea at MTD.
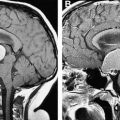
The role of hydroxyurea in preserving organ function in SCD is not yet determined. From a practical standpoint, these beneficial effects are difficult to assess prospectively because organ damage develops broadly over the whole pediatric age range, beginning with splenic and renal changes in infancy and evolving to pulmonary and neurologic deficits with vasculopathy among older children. In the Hydroxyurea Safety and Organ Toxicity (HUSOFT) study, infants with HbSS tolerated open-label liquid hydroxyurea and had preserved splenic filtrative function compared with historical controls. A follow-up study on this cohort identified preservation of splenic function and apparent gain of function in some cases. In a recent retrospective study, 43 children with HbSS had splenic function measured before and during treatment with hydroxyurea for a median duration of 2.6 years; six patients (14%) completely recovered splenic function and two (5%) had preserved splenic function, suggesting that hydroxyurea might help preserve or recover splenic function. Similar beneficial effects of hydroxyurea are reported anecdotally for children who have proteinuria, priapism, or hypoxemia.
The role of hydroxyurea in the prevention of stroke in SCD is an area of active investigation. In a retrospective study, hydroxyurea therapy was associated with lower transcranial Doppler (TCD) flow velocities. In a recent prospective single institution study, hydroxyurea was shown to decrease elevated TCD velocities significantly, often into the normal range, suggesting that hydroxyurea might serve as an alternative to chronic erythrocyte transfusions for primary stroke prophylaxis. Hydroxyurea also is reported as an alternative to chronic transfusions for secondary stroke prophylaxis in children for whom transfusions cannot be continued safely (eg, erythrocyte allosensitization). Hydroxyurea in combination with serial phlebotomy effectively prevented secondary stroke and led to resolution of transfusional iron overload in 35 children from a single institution. Based on these encouraging preliminary results, the NHLBI-sponsored Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) trial is underway ; this study randomizes children with previous stroke to standard therapy (transfusions and chelation) or alternative therapy (hydroxyurea and phlebotomy) for the prevention of secondary stroke and management of iron overload.
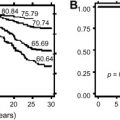
There are limited data regarding the prolonged use of hydroxyurea in SCD, particularly with regard to its long-term risks and benefits, but current clinical experience has not identified any clear detrimental effects or safety concerns. The possibility of hydroxyurea having negative effects on growth and development in children has not been realized. Similarly, concerns about DNA damage and leukemogenesis are not validated, with more than 15 years of exposure among adults and more than 12 years of exposure among children; continued vigilance is warranted but current data are encouraging regarding the long-term safety of this therapy. A 9-year observational follow-up study suggests that adults taking hydroxyurea had a significant 40% reduction in overall mortality. During the MSH Patients’ Follow-up study, there was little risk associated with the careful use of hydroxyurea; however, it was stated that hydroxyurea must be taken indefinitely to be effective and concerns remain over long-term safety. The teratogenicity of hydroxyurea for SCD is not elucidated fully. Anecdotes of normal offspring of women taking hydroxyurea during pregnancy are supported by the lack of birth defects observed in the MSH cohort (Abdullah Kutlar, personal communication, December 2007). Recent reports, however, document abnormal spermatogenesis in men taking hydroxyurea, so further investigation in this area is needed. A recently opened study at St Jude Children’s Research Hospital, entitled Long Term Effects of Hydroxyurea Therapy in Children with Sickle Cell Disease, should provide important data regarding long-term risks and benefits of hydroxyurea in this young patient population.
Clinical experience
Preclinical studies in anemic cynomolgus monkeys showed that hydroxyurea increased HbF levels. Pilot trials in patients with SCD demonstrated that hydroxyurea also increased HbF in humans and caused little short-term toxicity. These proof-of-principle experiments were critical first steps toward an important multicenter phase I/II trial involving adults with HbSS, which identified the short-term efficacy and toxicities of hydroxyurea used at maximum tolerated dose (MTD).
Developed on the basis of favorable results from the phase I/II trial, the National Heart, Lung, and Blood Institute (NHLBI) sponsored the pivotal Multicenter Study of Hydroxyurea (MSH), a double-blinded, placebo-controlled, randomized control trial conducted from 1992 to 1995 in 21 centers in the United States. and Canada. Two hundred and ninety-nine adult patients with HbSS were randomized (152 on hydroxyurea and 147 received placebo) but because of the beneficial effects observed, the trial was stopped early and only 134 subjects completed the planned 24 months of treatment. The hydroxyurea-treated subjects had a 44% reduction in painful crises per year (2.5 events per year versus 4.5 events per year) and a 58% reduction in median annual hospitalization rate for painful crisis (1.0 versus 2.4). In addition there were significantly fewer hydroxyurea-treated subjects who developed acute chest syndrome (ACS) (25 versus 51) and who received blood transfusions (48 versus 73); the number of units of blood transfused also was significantly less (336 versus 586). The incidence of death and stroke did not differ between the two treatment arms; there were no deaths related to hydroxyurea treatment and none of the patients who had received hydroxyurea developed cancer during the trial. The study did not address long-term safety or potential reversibility or prevention of chronic organ damage.
The results of this study led the Food and Drug Administration in 1998 to add to the indications for hydroxyurea, “to reduce the frequency of painful crises and to reduce the need for blood transfusions in adult patients with sickle cell anemia with recurrent moderate to severe painful crises.” This additional labeling refers only to adults severely affected by painful events rather than the broader spectrum of patients with SCD. Now, 10 years later, there is no change in the manufacturer’s drug labeling for hydroxyurea. Therefore, at this time, all children or patients with mild-to-moderate disease severity or those who do not have painful events but who have ACS or end-organ damage require off-label usage.
The initial success of hydroxyurea in adults led to the first pediatric multicenter phase I/II trial, known as HUG-KIDS, from 1994 to 1996. Eighty-four children ages 5 to 15 years with severe HbSS disease (defined as three or more painful events within the year before entry, three episodes of ACS within 2 years of entry, or three episodes of ACS or pain within 1 year of entry) were enrolled. Sixty-eight reached MTD and 52 were treated at MTD for 12 months. Similar hematologic effects were seen as in the MSH trial with decreased hemolysis (increased Hb and decreased reticulocytosis, decreased lactate dehydrogenase, and decreased total bilirubin), macrocytosis, improved erythrocyte hydration, myelosuppression, and increased HbF and F cells ( Table 1 ). Laboratory toxicities were mild and reversible with temporary interruption of the medication, and no life-threatening clinical adverse events were observed. Subsequent evaluation of this cohort revealed no adverse effect on height or weight gain or pubertal development. Predictors of HbF response were complex, but a higher treatment HbF was associated with higher baseline HbF, Hb, white blood cell count (WBC), and reticulocytes and compliance.
| Adults | Children | |
|---|---|---|
| MTD (mg/kg/d) | 21.3 | 25.6 |
| Δ Hb (g/dL) | +1.2 | +1.2 |
| Δ MCV (fL) | +23 | +14 |
| Δ HbF (%) | +11.2 | +9.6 |
| Δ Reticulocytes (10 9 /L) | −158 | −146 |
| Δ WBC (10 9 /L) | −5.0 | −4.2 |
| Δ ANC (10 9 /L) | −2.8 | −2.2 |
| Δ Bilirubin (mg/dL) | −2.0 | −1.0 |
Short-term clinical efficacy in children initially was reported in small open-label studies. In a small, randomized study from Belgium, children with HbSS treated with hydroxyurea had significantly fewer hospitalizations for pain, with shorter lengths of stay, compared with those receiving placebo. Additional European data showed improved laboratory and clinical response without significant toxicity and no growth or pubertal delay. Follow-up studies have revealed continued efficacy in association with long-term hydroxyurea use in children, including a sustained HbF response greater than 20% using hydroxyurea at MTD.
The role of hydroxyurea in preserving organ function in SCD is not yet determined. From a practical standpoint, these beneficial effects are difficult to assess prospectively because organ damage develops broadly over the whole pediatric age range, beginning with splenic and renal changes in infancy and evolving to pulmonary and neurologic deficits with vasculopathy among older children. In the Hydroxyurea Safety and Organ Toxicity (HUSOFT) study, infants with HbSS tolerated open-label liquid hydroxyurea and had preserved splenic filtrative function compared with historical controls. A follow-up study on this cohort identified preservation of splenic function and apparent gain of function in some cases. In a recent retrospective study, 43 children with HbSS had splenic function measured before and during treatment with hydroxyurea for a median duration of 2.6 years; six patients (14%) completely recovered splenic function and two (5%) had preserved splenic function, suggesting that hydroxyurea might help preserve or recover splenic function. Similar beneficial effects of hydroxyurea are reported anecdotally for children who have proteinuria, priapism, or hypoxemia.
The role of hydroxyurea in the prevention of stroke in SCD is an area of active investigation. In a retrospective study, hydroxyurea therapy was associated with lower transcranial Doppler (TCD) flow velocities. In a recent prospective single institution study, hydroxyurea was shown to decrease elevated TCD velocities significantly, often into the normal range, suggesting that hydroxyurea might serve as an alternative to chronic erythrocyte transfusions for primary stroke prophylaxis. Hydroxyurea also is reported as an alternative to chronic transfusions for secondary stroke prophylaxis in children for whom transfusions cannot be continued safely (eg, erythrocyte allosensitization). Hydroxyurea in combination with serial phlebotomy effectively prevented secondary stroke and led to resolution of transfusional iron overload in 35 children from a single institution. Based on these encouraging preliminary results, the NHLBI-sponsored Stroke With Transfusions Changing to Hydroxyurea (SWiTCH) trial is underway ; this study randomizes children with previous stroke to standard therapy (transfusions and chelation) or alternative therapy (hydroxyurea and phlebotomy) for the prevention of secondary stroke and management of iron overload.
There are limited data regarding the prolonged use of hydroxyurea in SCD, particularly with regard to its long-term risks and benefits, but current clinical experience has not identified any clear detrimental effects or safety concerns. The possibility of hydroxyurea having negative effects on growth and development in children has not been realized. Similarly, concerns about DNA damage and leukemogenesis are not validated, with more than 15 years of exposure among adults and more than 12 years of exposure among children; continued vigilance is warranted but current data are encouraging regarding the long-term safety of this therapy. A 9-year observational follow-up study suggests that adults taking hydroxyurea had a significant 40% reduction in overall mortality. During the MSH Patients’ Follow-up study, there was little risk associated with the careful use of hydroxyurea; however, it was stated that hydroxyurea must be taken indefinitely to be effective and concerns remain over long-term safety. The teratogenicity of hydroxyurea for SCD is not elucidated fully. Anecdotes of normal offspring of women taking hydroxyurea during pregnancy are supported by the lack of birth defects observed in the MSH cohort (Abdullah Kutlar, personal communication, December 2007). Recent reports, however, document abnormal spermatogenesis in men taking hydroxyurea, so further investigation in this area is needed. A recently opened study at St Jude Children’s Research Hospital, entitled Long Term Effects of Hydroxyurea Therapy in Children with Sickle Cell Disease, should provide important data regarding long-term risks and benefits of hydroxyurea in this young patient population.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree