Fig. 1.1
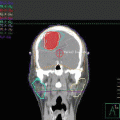
Pre-chemotherapy PET/CT scan (axial slices) demonstrating the extent of disease in the right neck
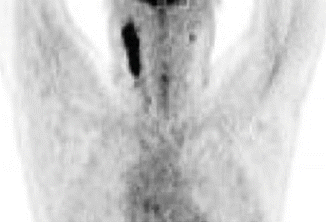
Fig. 1.2
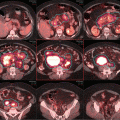
Pre-chemotherapy PET/CT scan (coronal view) demonstrating the extent of disease
Pathology
The final pathology demonstrated nodular sclerosis (classic) HL (NSHL). NSHL is composed of Reed–Sternberg (R–S) cells (CD15+) identified in a background of predominantly lymphocytes. NSHL is characterized by collagenous bands that divide the lymph node into nodules often containing an R-S cell variant called the lacunar cell. Although NSHL is the most common subtype in all age groups, it is more frequent in adolescents (77 %) and adults (72 %) than in younger children (44 %) [1]. NSHL frequently spreads along contiguous nodal chains as illustrated in this case presentation in which the right neck was involved extending from lymph node levels II–IV.
Staging and Prognostic Factors
After pathology is obtained, patients undergo clinical staging, beginning with a detailed history of systemic symptoms and physical examination. Laboratory studies including CBC and biochemical evaluation with liver function tests (including albumin) should be obtained. Acute-phase reactants, including ESR and C-reactive protein, may be elevated at diagnosis. Patients with “B” symptoms or stage III and IV lymphoma should have a bone marrow biopsy. Given this patient presented with stage IA disease, he did not undergo bone marrow biopsy. Imaging work-up with CT scan of the neck, chest, abdomen, and pelvis with IV contrast is required to define disease involvement and extent [1]. An abdominal and pelvic CT with oral and intravenous contrast can define retroperitoneal and pelvic lymph nodes. HL involving the liver or spleen is suggested by CT findings of definite areas of abnormal density representing lymphomatous deposits. 18F-FDG PET/CT scan is increasingly recognized as the most useful functional staging modality for HL [2]. Areas of abnormal avidity correlate with disease extent, which is essential in staging, and PET scan is critical for assessment of mid-treatment and end-of-treatment response. PET scan, when performed after only two or three cycles of chemotherapy, has prognostic significance in classical HL whether in the initial or relapsed setting [3–5]. A negative mid-treatment PET has been clearly associated with favorable outcomes. While HL patients with a positive mid-treatment PET generally fare worse, the results have been variable given the small number of PET-positive patients seen in these studies. Early metabolic imaging is also being investigated in an effort to reduce chemotherapy toxicity in adequately responding patients while intensifying patients who are slow to respond [6]. Large-scale clinical trials have also used end-of-chemotherapy PET to guide whether and which sites to radiate particularly in the advanced HL setting [7, 8]. There has been a movement to carefully define responses objectively, and ongoing trials and clinical guidelines have used the five-point Deauville criteria based on the mediastinal blood pool and liver as internal benchmarks of uptake [9]. Information gained on the extent of early and end-of-chemotherapy response may help to individually tailor risk-adapted, combined-modality treatment for HL. Currently, and for patients with early favorable HL, HD10 still constitutes the backbone.
In addition to the emerging data on the prognostic significance of response-based imaging, there are several well-established prognostic factors including the stage of disease, presence of bulk, number of involved sites, systemic “B” symptoms, laboratory studies including ESR, and age. Patients presenting with advanced disease (stage III or IV) have a poorer outcome than patients with early-stage disease [10]. “B” symptoms develop as a result of cytokine secretion and are a marker for biologic aggressiveness of disease. ESR, hemoglobin level, and serum albumin have also been reported to predict worse outcomes [11]. HL is typically categorized into “favorable” and “unfavorable” disease based on the constellation of such prognostic factors, and each large cooperative group has defined these categories with specific criteria (See Table 1.1). Therefore, in interpretation of clinical trial data, it is essential to understand the inclusion criteria for “favorable” and “unfavorable” categories to apply the clinical outcomes appropriately.
Table 1.1
Definitions of unfavorable risk factors by a specific cooperative group
German Hodgkin Study Group (GHSG) |
1. ESR >50 if no B symptoms, ESR >30 if B symptoms |
2. Mediastinal mass ratio >0.33 |
3. >2 nodal sites |
4. Any extranodal disease |
European Organization for the Research and Treatment of Cancer (EORTC) |
1. Age ≥50 |
2. ESR >50 if no B symptoms, ESR >30 if B symptoms |
3. Mediastinal thoracic ratio >0.35 |
4. >3 nodal sites |
National Cancer Institute of Canada (NCIC) |
1. Age ≥40 |
2. Mixed cellularity or lymphocyte depleted histology |
3. ESR >50 or any B symptoms |
4. Mediastinal mass ratio >0.33 or disease >10 cm |
5. >3 nodal sites |
Our patient presented at a young age, without B symptoms or bulk, with disease confined to one “site” in the right neck, and normal lab values. Therefore, he fell classically into a “favorable” category of disease according to each cooperative group’s definition.
Treatment Management
Randomized controlled data have demonstrated the feasibility of lowering the radiation prescription dose particularly in the favorable group of patients. The German Hodgkin Study Group (GHSG) HD10 trial used a 2 × 2 design to randomize patients to either two or four cycles of ABVD chemotherapy with either 20 Gy- or 30 Gy involved-field radiotherapy (IFRT). There were no significant differences in overall survival and a 5-year freedom-from-treatment failure based on the number of chemotherapy cycles or radiation dose [12]. It is important to note that the GHSG definition of favorable disease differs from both the EORTC and National Cancer Institute of Canada (NCIC) definitions (Table 1.1). In this patient, ABVD was recommended with two cycles followed by 20 Gy of radiation treatment because he met favorable disease criteria per the HD10 trial.
Concerns regarding late radiation toxicity have prompted interest in exploring the feasibility of a chemotherapy-alone approach. A number of trials have compared combined modality therapy with chemotherapy alone, and these studies can be difficult to interpret due to varying inclusion criteria and treatment techniques. Randomized comparisons of combined modality therapy with chemotherapy alone generally show higher rates of relapse with omission of RT. The EORTC-GELA H9-F examined 619 patients with early-stage, favorable disease who had achieved a complete response after six cycles of epirubicin, bleomycin, vinblastine, and prednisone (EBVP) chemotherapy [13]. Following chemotherapy, patients were randomized to observation, 20 Gy of IFRT or 36 Gy of IFRT. There was no difference in a four-year EFS between the two IFRT arms (20 Gy: 84 % vs. 36 Gy: 87 %), and patients in the observation arm experienced a significantly lower four-year EFS of 70 % (p < 0.001) which resulted in early termination of the observation arm due to prespecified stopping rules. It has been noted that the H9F study employs less intensive chemotherapy than other similarly designed trials, and it has been suggested that this is the reason for a lower EFS in the observation arm [14].
Similarly, in the National Cancer Institute of Canada – Eastern Cooperative Oncology Group (NCIC-ECOG) HD6 trial, 405 patients with early-stage disease were randomized to four to six cycles of ABVD chemotherapy alone versus subtotal nodal irradiation (STNI) with either two cycles of ABVD chemotherapy or no further therapy. Patients in the combined modality arm experienced a trend toward an improved 12-year FFDP compared to patients in the chemotherapy alone arm (92 % vs. 87 %, p = 0.05), with “unfavorable” patients experiencing a statistically significant difference in FFDP (94 % vs. 86 %, p = 0.01) [15]. Notably, patients in the combined modality arm experienced worse OS (87 % vs. 94 %, p = 0.04). However, interpretation of the survival results is unclear given an excess of mortality in the combined modality arm from either early toxicity or causes other than lymphoma. Ultimately, the omission of radiation did not adversely affect OS rates in the majority of patients on the HD6 trial. It is critical to be aware that the HD6 trial incorporated more extensive RT with STNI than is delivered with modern radiotherapy. Although the combined modality arm in this study is obsolete, enthusiasts of a chemotherapy-only approach have pointed to the excellent survival results with four to six cycles of ABVD [14]. Therefore, the outcomes of chemotherapy, compared to combined modality therapy delivered with modern techniques, smaller radiation fields, and lower doses, remain to be determined.
A systematic Cochrane review further investigated the omission of RT from combined modality therapy. The analysis included five unconfounded trials and demonstrated that the omission of RT from the combined modality paradigm led to increased risk of relapse [16]. Additionally, two recent analyses using large-scale datasets from the Surveillance, Epidemiology, and End Results (SEER) program and the National Cancer Database have also suggested decreased overall survival in early stage HL patients treated with chemotherapy alone [17, 18].
Excellent survival outcomes and availability of effective salvage therapy do suggest that chemotherapy alone is an option particularly in those patients that are likely to experience morbidity from the late effects of RT. However, there are trade-offs with this approach as it requires more intensive chemotherapy or increased number of chemotherapy cycles, and in the case of relapse, higher doses of RT and/or stem cell transplantation are required resulting in more significant toxicities. To better determine the patients in which to escalate or de-escalate therapy, studies are ongoing to evaluate whether excellent clinical outcomes can be maintained with the omission of RT after a negative interim PET. Although this strategy holds promise, it is not clear yet whether there is predictive power to the interim PET that will guide risk-adapted therapy in the adult population. For example, favorable patients in the H10 trial that had a negative PET after ABVD x 2 cycles received one additional cycle of ABVD without RT. This experimental arm was closed early due to excess treatment failure, and RT was subsequently added to this arm following ABVD completion. There were 16 events of progression in the experimental arm versus 7 in the standard arm at first interim analysis, and the data-monitoring committee concluded that it was unlikely that the experimental arm would have a non-inferior outcome leading to the early closure [19]. The United Kingdom National Cancer Research Institute RAPID trial also explored the role of interim PET in patients with stage I/II disease without B symptoms or mediastinal bulk. Patients with a negative PET scan following three cycles of ABVD were randomized to IFRT versus observation. The trial was designed as a non-inferiority study, and at a median follow-up of 45.7 months, the 3-year PFS was similar between the IFRT and observation arms (IFRT 93.8 vs. observation 90.7 %). However, the 95 % confidence interval for the difference in PFS exceeded the prespecified non-inferiority boundary of 7 %. Additionally, a number of patients randomized to the IFRT arm did not actually receive RT, and therefore, a secondary per-protocol analysis was performed, which showed a significant improvement in PFS with IFRT (3 years PFS with RT: 97.1 % vs. no RT: 90.8 %, p = 0.02). The results of this trial are therefore difficult to interpret given the similarity in PFS on the intent-to-treat analysis with a significant difference demonstrated on the per-protocol analysis [20].
Treatment Field and Technique
With the advent of modern chemotherapy, IFRT became the standard radiation technique based on randomized trial data supporting the use of IFRT over an EFRT approach in the context of chemotherapy. Because patients with early-stage HL treated with chemotherapy alone most frequently relapse in the initially involved lymph node(s) [21], efforts have been made to reduce treatment fields to include only the initially involved lymph node(s) and exclude surrounding normal tissues. The EORTC-GELA introduced the concept of INRT [22], which uses all available clinical information, including pre- and post-chemotherapy imaging with CT and FDG-PET scan to define the treatment field. The application of INRT relies on a pre-chemotherapy PET/CT scan to be performed in the treatment position to facilitate fusion with the simulation scan.
However, it is a common scenario that pre-chemotherapy imaging is not available and has not been performed in the treatment position. As a result, the involved site radiation therapy (ISRT) guidelines were developed and similarly target the initially involved lymph nodes, allowing for a potentially larger margin based on the uncertainty introduced by the lack of pre-chemotherapy imaging or inability to fuse such imaging accurately [23]. ISRT fields were developed to take into consideration modern technology with the use of staging PET/CT scans, 3D, and 4D CT-based treatment planning, conformal treatment techniques, and the use of image guidance in an effort to replace the approach of IFRT which was based on 2D treatment planning and bony anatomy.
In this patient, ISRT was utilized to target the initially involved lymph nodes (Fig. 1.3). A pre–chemotherapy GTV was constructed to identify the areas of initial involvement. The patient had an excellent response to chemotherapy with no evidence of FDG-avid disease noted on their post–chemotherapy PET/CT scan. Although a PET complete response was achieved, there was evidence of post–chemotherapy residual CT abnormality that was contoured as the post–chemotherapy GTV. The CTV was then designed to encompass both the superior and inferior extent but not axial direction of the pre–chemotherapy GTV (Fig. 1.2). The CTV was modified to exclude normal anatomy and adjusted to take into account the change in normal anatomic position of the surrounding normal tissues. The CTV was expanded volumetrically to a margin of 5 mm to a final PTV, keeping in mind that this is a neck site with reproducible immobilization, as opposed to a mediastinal location for instance, and therefore does not need as generous of an expansion (Figs. 1.3 and 1.4).
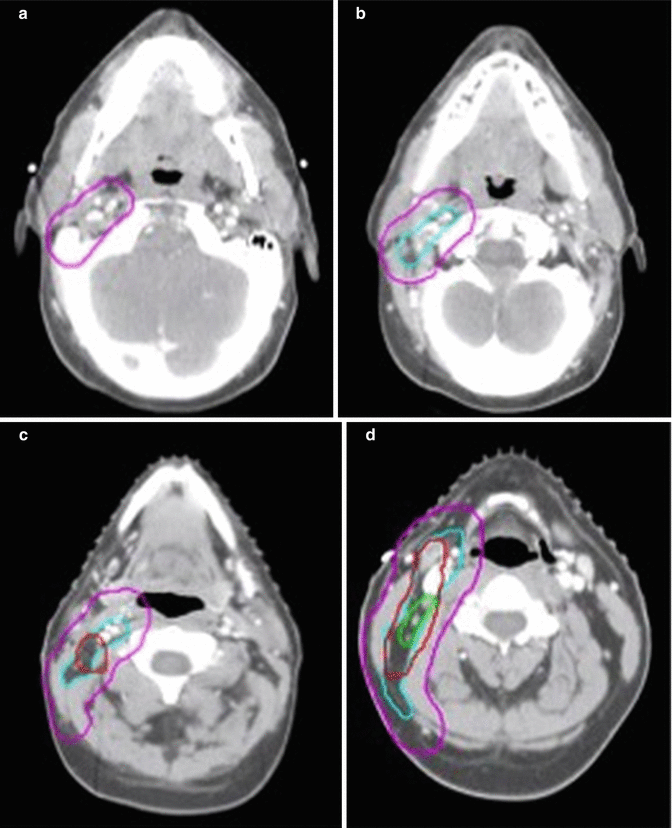
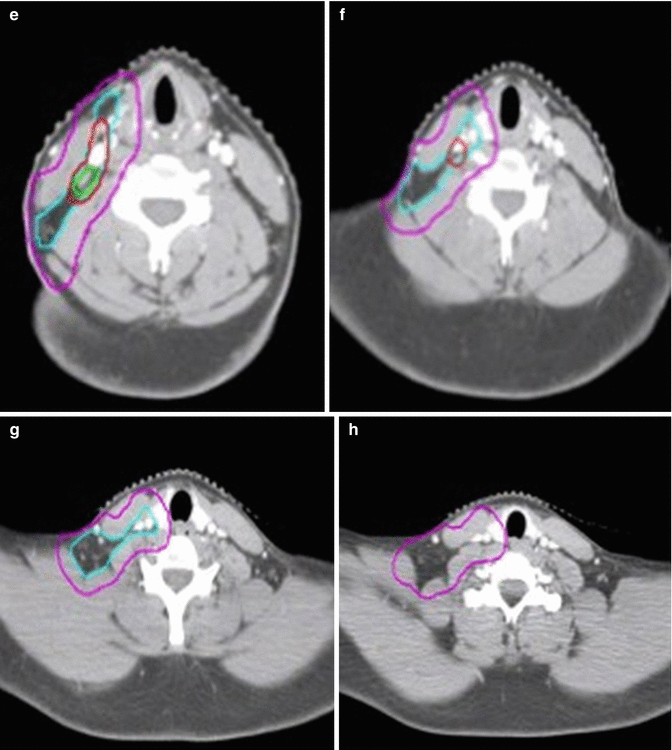
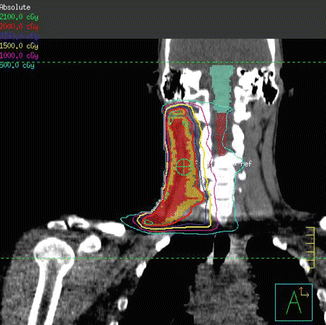


Fig. 1.3
CT simulation scan with contours (axial slices). Pre-chemotherapy GTV (red), post-chemotherapy GTV (green), CTV (light blue), PTV (pink)

Fig. 1.4
Intensity-modulated radiation therapy plan demonstrating coverage of the CTV (shaded mustard) by the 100 % isodose line (red)
Low dose radiation delivered to a final dose of 20 Gy in 2 Gy fractions was planned using 3D conformal therapy. Beams were oriented in an AP/PA direction with wedges placed to improve dose homogeneity (Fig. 1.5).
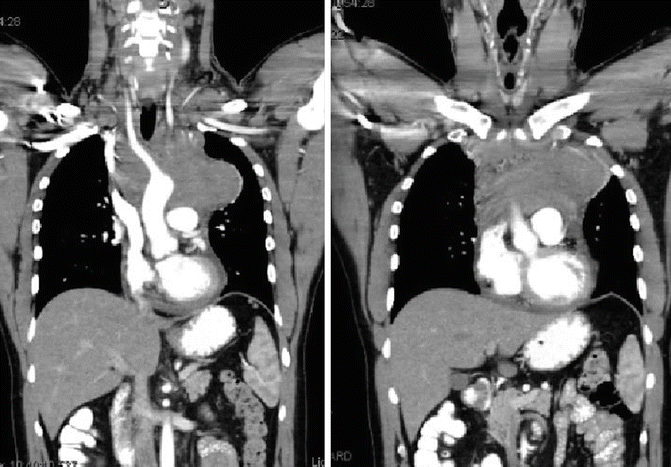

Fig. 1.5
Showing coronal pre-chemotherapy CT images, note the bilateral neck disease, bulky mediastinal mass, and the pericardial effusion
Posttreatment Considerations
Given the evolution of RT field design in the reduction of field size and dose, it is difficult to interpret the current body of literature on RT-related late toxicities, which is heavily derived from patients who were treated with EFRT techniques. The patient described here, reported grade 1 fatigue and grade 1 esophagitis during the course of radiotherapy. At 2 years posttreatment, he has reported no sequelae with resolution of his fatigue as well as esophagitis. Long-term follow-up evaluating thyroid function tests will be important given the location of ISRT.
Long-term follow-up for such patients will be critical to understand how the reduction in field sizes using ISRT and INRT translates in terms of the development of late toxicities [24]. Additionally, initial data on the use of smaller field sizes has suggested that clinical outcomes are not sacrificed with the use of INRT or ISRT. Paumier et al., for example, reported a 92 % 5-year PFS in patients with early-stage HL treated with INRT as per EORTC-GELA guidelines [25]. Similarly, Filippi et al. reported a 3-year relapse-free survival of 99 % in stage IIA patients using an ISRT technique [26]. The current GHSG HD17 trial is open for newly diagnosed intermediate stage Hodgkin lymphoma, delivering two cycles of escalated BEACOPP and two cycles of ABVD followed by assessment of FDG-PET scan. Patients randomized into the standard arm receive IFRT regardless of PET response. Patients randomized into the experimental arm receive no further therapy if their PET is negative and receive INRT in patients if their PET scan is positive.
Clinical Presentation: Combined Modality Therapy for Early-Stage Unfavorable Patients
A 28–year-old man presented with dyspnea on exertion and pleuritic chest pain. Further work–up led to a chest X–ray which revealed mediastinal widening. A chest CT showed an anterior mediastinal mass measuring 9.5 × 5.0 × 4.5 cm, bilateral supraclavicular adenopathy, and presence of pericardial effusion (see Fig. 1.5). Excisional biopsy of a supraclavicular node showed classical NSHL.
Staging and Prognostic Factors
The patient’s history was notable for a 2–month progressive dyspnea on exertion, but absence of B symptoms or signs of superior vena cava syndrome. His physical examination showed a well–healing biopsy scar in the left supraclavicular area and slight fullness in the right supraclavicular area. He has no other sites adenopathy and no organomegaly. Laboratory studies showed a normal CBC with differential, and his ESR was 12 mm/h. PET/CT scan confirmed FDG–avid disease limited to the bilateral supraclavicular fossa and anterior mediastinum (see Fig. 1.6). Although a pericardial effusion was noted, there was no pericardial nodal disease or disease below the diaphragm.
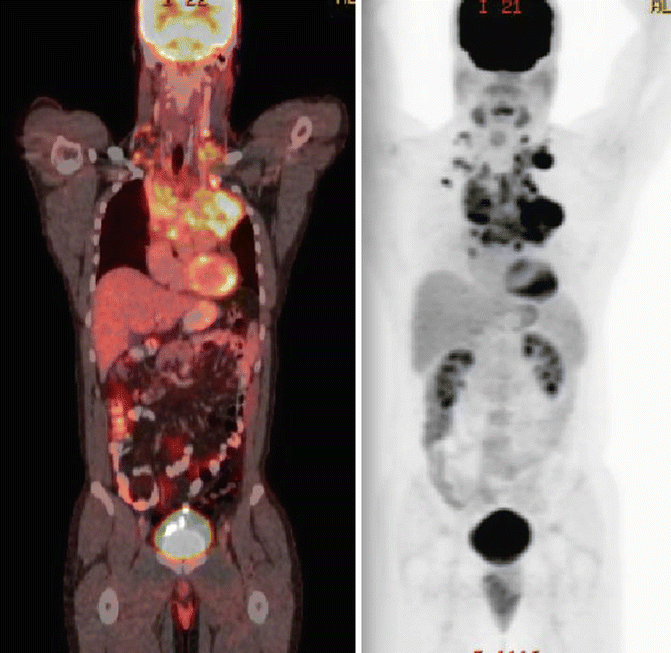

Fig. 1.6
Showing the original disease on the pre-chemotherapy PET/CT
This patient did not have B symptoms and his laboratory findings are within normal limits. He did not have bulky disease since his mediastinal mass ratio on chest X–ray is <0.33, and his maximal disease dimension is <10 cm. However, he did have three sites of disease (bilateral supraclavicular and mediastinal disease) and is therefore classified as unfavorable according to the GHSG criteria but favorable by the EORTC criteria.
Treatment Management
In the treatment management of early-stage unfavorable-prognosis patients, investigators have explored chemotherapy regimens other than ABVD, the optimal duration of chemotherapy, and optimal radiation dose. Both the EORTC and HD11 studies H9U [27, 28] studies compared 4–6 cycles of ABVD with four cycles of BEACOPP-baseline, followed by involved-field radiation therapy to 20–30 Gy. No significant differences in a 4-year event-free survival (EFS) or overall survival were observed between BEACOPP and ABVD in the EORTC H9U trial. In the GHSG HD11 trial, although there was a significantly higher 5-year freedom-from-treatment failure (FFTF) in the four cycles of baseline BEACOPP arm over the four cycles ABVD arm if followed by 20 Gy of involved-field radiation therapy, no difference was observed, and 30 Gy of involved-field radiation therapy was used. Patients treated with baseline BEACOPP had a higher rate of severe toxicity. The GHSG HD14 trial [29] compared four cycles of ABVD with two cycles of dose-escalated BEACOPP and two cycles of ABVD (2 + 2 regimen), followed by involved-field irradiation to 30 Gy. Although the 2 + 2 regimen had a significantly improved 5-year FFTF, it was associated with more acute toxicity, and there was no overall survival advantage. In a subset analysis of E2496 trial of patients with stage I–II bulky disease, there was no significant difference in a 5-year failure-free, and overall survival rates between ABVD and radiotherapy versus Stanford V [30]. Given these data, ABVD remains the most widely accepted chemotherapy regimen for patients with early-stage, unfavorable disease in North America. The question of optimal chemotherapy duration was addressed by the EORTC-H8U and EORTC-H9U trials [27, 31], both showing no difference in EFS between four versus six cycles of chemotherapy, suggesting that in early-stage unfavorable patients, four cycles of ABVD is adequate. However, it should be noted that the GSHG HD11 excluded patients with stage IIB with large mediastinal adenopathy or extranodal disease. Instead, these patients were included in their advanced-stage trials. For patients with early-stage disease with multiple risk factors, a longer course of chemotherapy should therefore be considered.
The question of optimal radiation dose in unfavorable patients was addressed by the previously described GHSG HD11 trial [28] with a 2 × 2 design comparing 20 Gy versus 30 Gy after four cycles of ABVD or baseline BEACOPP. At a median follow-up of 82 months, the 5-year freedom-from-treatment-failure rates were 81.1 %, 85.3 %, 86.8 %, and 87.0 %, respectively. There was no significant difference in a 5-year FFTF rate between baseline BEACOPP and ABVD when followed by 30 Gy of IFRT; however, inferiority of 20 Gy cannot be excluded after four cycles of ABVD, leading to the authors’ conclusion of four cycles of ABVD followed by 30 Gy of radiation therapy as optimal therapy for early-stage, unfavorable HL. Although the GHSG HD11 results do not support radiation dose de-escalation after ABVD in early-stage unfavorable patients, there are single institutional data showing that, especially if there is a complete metabolic response to chemotherapy, doses of 20–25 Gy may be adequate [32, 33].
Our patient has unfavorable disease according to the GHSG criteria, with having three sites of disease as his only risk factor. He received a total of four cycles of ABVD and achieved a complete response (Deauville 2). He then went onto receive ISRT to 30.6 Gy in 17 fractions.
Treatment Field and Technique
ISRT was utilized, targeting the initially involved lymph nodes, including the bilateral supraclavicular and mediastinal nodes, as detailed above. Figure 1.7a, b, shows the CTV and PTV on the planning CT and superimposed on the pre–chemotherapy CT. The pericardial effusion was not considered part of the CTV, since pericardial effusions in this setting are often reactive in nature, and the suspicion level for any residual microscopic disease is low. Furthermore, its inclusion as part of the target volume would substantially increase doses to the heart.
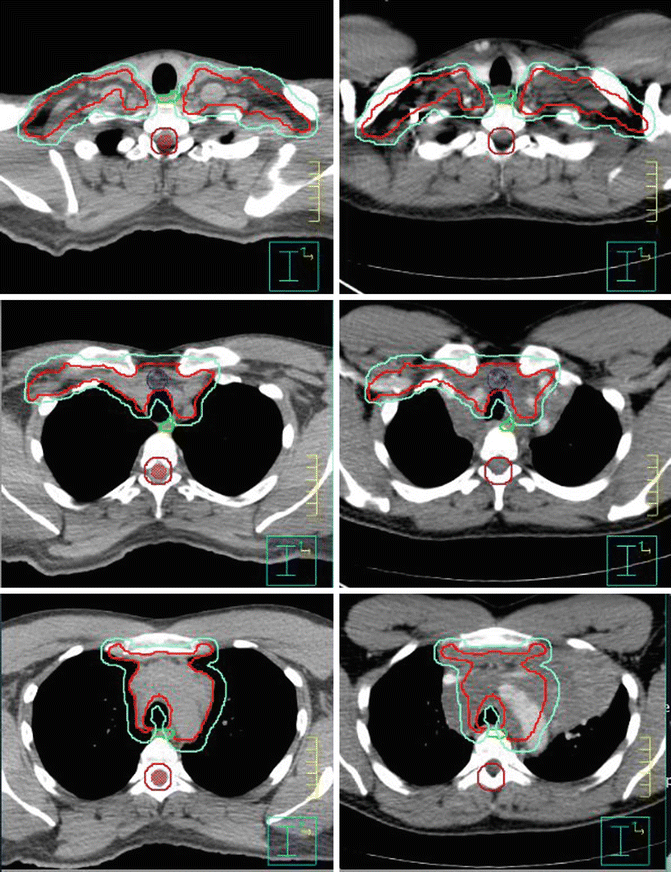

Fig. 1.7
Showing the CTV (red) and PTV (green). Panel of the left is the CT simulation; panel of the right is the fuse pre-chemotherapy images
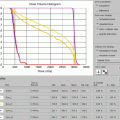
The patient was placed in an arms-down position (an arms-down position would be especially important in a young woman to limit the amount of breast tissue in the treatment field). The patient was planned with the deep inspiration breath–hold (DIBH) technique, which has been shown to significantly reduce doses to the lungs, heart, and cardiac substructures. The butterfly IMRT technique of using anterior beams of 300°–30° and posterior beams of 160–210° was used to limit the low-dose bath laterally. Coronal and axial images of the isodose distribution in Fig. 1.8a, b, show the sparing of the majority of the heart. The DVHs are displayed in Fig. 1.9. With the butterfly IMRT technique in DIBH position, a mean heart dose of 5.4 Gy and mean lung dose of 8.8 Gy were achieved. A mean dose to the left coronary artery was 7.1 Gy.
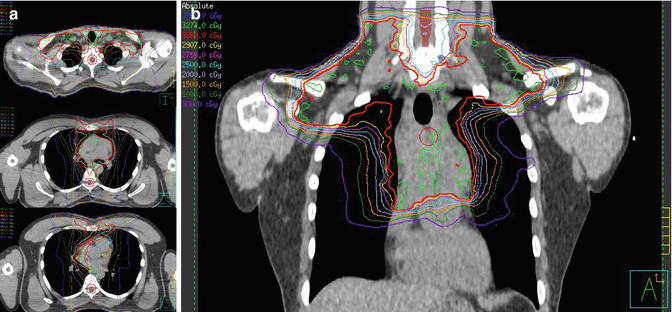
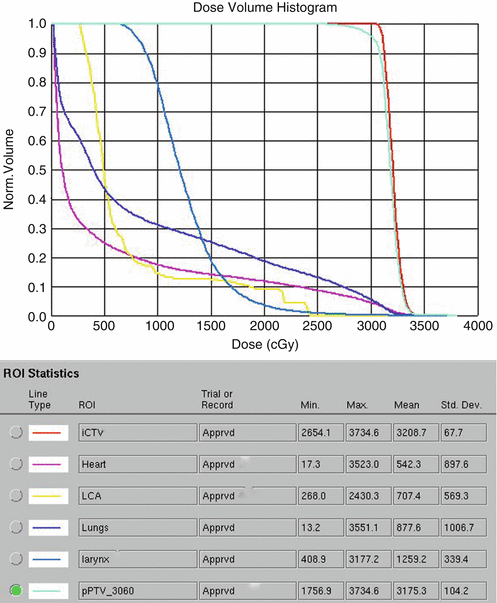

Fig. 1.8
(a) Showing the isodose lines, 100 % in red covering PTV and avoiding left ventricle as well as left coronaries. (b) Coronal view of the isodose lines, low-dose bath is limited by using butterfly technique. The heart and its structure dose is also limited by using IMRT

Fig. 1.9
Showing dosimetric values of the organs at risk especially the total lung (blue) and heart (pink)
Posttreatment Considerations
The patient experienced esophagitis and mild fatigue toward the end of the treatment course. At 1–month follow–up, these symptoms largely resolved. As part of follow–up, the patient will undergo annual thyroid function tests. The long–term cardiac risk in this patient is expected to be significantly lower than patients who were treated with full mantle field to higher doses of radiation. Nevertheless, he will benefit from close monitoring of and minimizing traditional cardiac risk factors, including hypertension, hyperlipidemia, and diabetes, and other lifestyle modifications such as healthy diet, regular exercise, and avoiding tobacco use. A baseline cardiology evaluation including resting and stress echocardiogram should be considered approximately 10 years posttreatment. If he were to have had a tobacco history, an annual low–dose chest CT screening for lung cancer should be considered approximately 10 years posttreatment.
Clinical Presentation: Refractory and Relapsed Disease
This patient is a 37-year–old man who was initially diagnosed with upper mediastinal classical HL in 2009. He was treated with ABVD chemotherapy x 6 cycles alone with no further adjuvant therapy. He recurred 6 months later in the mediastinum and received salvage therapy with ICE (e.g., ifosfamide, carboplatin, and etoposide) followed by busulfan/cytoxan preparation regimen with autologous peripheral stem cell transplant shortly thereafter and subsequently attained a CR. Three years later he had a CT scan for follow–up that demonstrated an enlarging mediastinal lesion in the upper mediastinum. A PET/CT demonstrated a 3 × 1.9 cm lobulated nodal conglomerate in the superior mediastinum anterior to the upper trachea with an SUV of 11.5 with inferior extension into the retrosternal anterior mediastinum measuring 3.7 × 1.5 cm (Fig. 1.10). No other disease was identified. The patient underwent core biopsy through an endobronchial approach (EBUS) that confirmed disease recurrence.
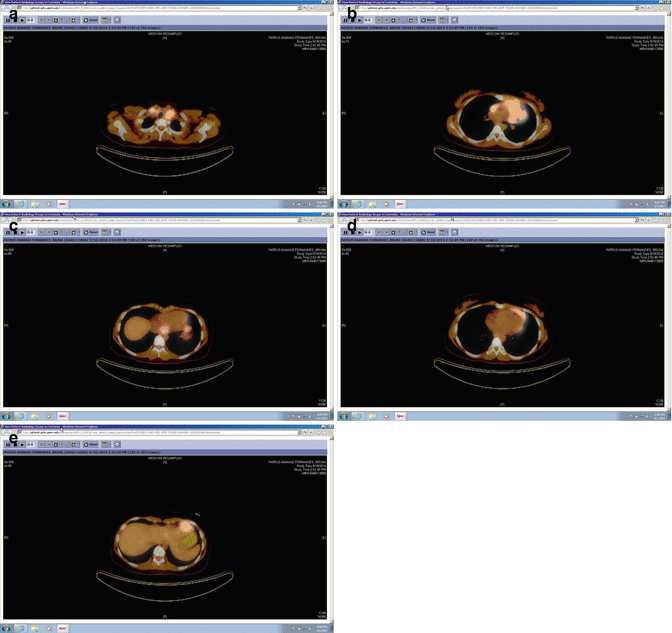

Fig. 1.10
Pre-chemotherapy PET/CT scan (axial slices) demonstrating the extent of disease in the upper mediastinum
Pathology, Staging, and Prognostic Factors
Pathology at the time of relapse demonstrated numerous small mature lymphocytes with rare binucleate cells with prominent nucleoli seen. A panel of immunostains demonstrated that the large atypical cells are CD30 and CD15. The background lymphocytes are predominantly CD20-positive B cells. In situ hybridization for EBV (EBER) was negative. The lack of a mixed inflammatory background was unusual, but the morphologic and immunophenotypic findings in the setting of this patient’s history were consistent with classical HL. Historically, clinical factors that independently predict for a more favorable outcome in the relapsed setting include nodal relapse site (as opposed to extranodal), early stage at relapse, and response to first–line salvage chemotherapy [1].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree