INTRODUCTION
Hodgkin lymphoma (HL) was recognized in the first half of the nineteenth century by Thomas Hodgkin and Samuel Wilks (1). HL usually arises in lymph nodes, preferentially in the cervical area, and the majority of HLs manifest clinically in young adults in their third and fourth decades of life. The incidence of HL is 3.0 per 100,000 person-year in the United States; it is higher in the Western countries than Asian countries (2,3). Biological and clinical studies have shown that HLs are comprised of two disease entities: nodular lymphocyte-predominant HL (NLPHL) and classical HL (cHL) (1). The two entities differ in their clinical features and behavior. Within cHL, four subtypes have been described: nodular sclerosis, mixed cellularity, lymphocyte-rich, and lymphocyte-depleted. These four subtypes differ in their clinical features, growth pattern, presence of fibrosis, and frequency of Epstein-Barr virus infection but share the immunophenotype of tumor cells.
The management of HL continues to evolve. Before the widespread use of modern polychemotherapy, large-field radiation therapy was able to cure cHL patients. However, reliance on radiation alone required extensive radiation portals to treat nearly the entire lymphatic system with radiation doses up to 44 Gy. With long-term follow-up, many patients developed heart toxicity and second malignancies. Therefore, efforts have been made to reduce the long-term toxicities of treatment for HL while maintaining excellent cure rates. With modern chemotherapy, multiple randomized studies have shown that radiation portals can be safely reduced from extended-field radiation to involved-field radiation and, now, even smaller fields of radiation, including involved-node radiation.
Currently, the treatment of cHL is stratified by risk groups—early-stage favorable, early-stage unfavorable, and advanced-stage disease—according to the clinical stage and the presence or absence of adverse clinical features. In this chapter, we will review advances in the management of HL, including recent publications about strategies of management of the rarer diagnosis of NLPHL.
TYPES OF HODGKIN LYMPHOMA
Over the past decade, investigators have made significant progress in the diagnosis, classification, staging, prognosis, and treatment of HL. In past years, the true lineage of the neoplastic cells in HL was unknown; hence, the term Hodgkin disease was used. It is now recognized that almost all cases of HL are of B-cell lineage; hence, the name changed to Hodgkin lymphoma.
The classification of HL has remained relatively stable over the past 40 years, and the World Health Organization (WHO) classification of lymphoid neoplasms was updated in 2008 (1) (Table 10-1). The current WHO classification recognizes that NLPHL is distinct from the other types that can be grouped together under the rubric of cHL (1).
In 2015, it was estimated that 9,050 Americans would be diagnosed with HL, with a median age at diagnosis of 39 years (4). Hodgkin lymphoma has been traditionally defined as a hematopoietic neoplasm composed of diagnostic Hodgkin and Reed-Sternberg (HRS) cells within a reactive cell background. An HRS cell is large, 30 to 60 μm, containing a bilobed vesicular nucleus, with each lobe containing a prominent round eosinophilic nucleolus surrounded by a clear zone or halo; it also has abundant cytoplasm. However, HRS cells often comprise less than 1% of the involved tumor tissue and are absent in NLPHL. Hodgkin and Reed-Sternberg cells are believed to be derived from germinal center (GC) B cells that have unfavorable immunoglobulin V gene mutations, whereas lymphocyte-predominant (LP) cells, which were previously termed lymphocytic and histiocytic (LH) cells, are thought to originate from antigen-selected GC B cells (5).
Approximately 5% of patients with HL have NLPHL. This disease is usually localized and most often involves cervical or axillary lymph nodes (1,6). The disease affects patients of all ages, males more often than females, and is clinically indolent (Table 10-2). Systemic symptoms—such as fever, weight loss, and nights sweats (also known as B symptoms)—are infrequent. Patients with NLPHL are characterized as having a more indolent course with delayed relapse compared with cHL, analogous to low-grade non-HL (6). Patients with NLPHL are at 5% to 6% risk for developing diffuse large B-cell lymphoma (DLBCL) or T-cell/histiocyte-rich large B-cell lymphoma (1).
| Clinical Feature | NLPHL | Classical HL |
|---|---|---|
| Frequency | 5% | 95% |
| Age distribution | Unimodal: equal in children and adults | Bimodal: peak in second and third decades |
| Male | 70% | 50% |
| Sites involved | Lymph nodes with sparing mediastinum | Mediastinum, cervical lymph nodes |
| Stage at diagnosisa | I | II or III |
| B symptoms | <20% | 40% |
| Clinical course | Indolent, late relapses | Aggressive, curable |
The German Hodgkin Lymphoma Study Group (GHSG) has reported a large retrospective study of 394 patients with NLPHL. Patients were predominantly men (75%), only 9% had B symptoms, and 79% had early-stage disease (7). With a median follow-up of 50 months, tumor control (freedom from treatment failure) and overall survival were 88% and 96%, respectively, slightly better than in cHL (82% and 92%, respectively).
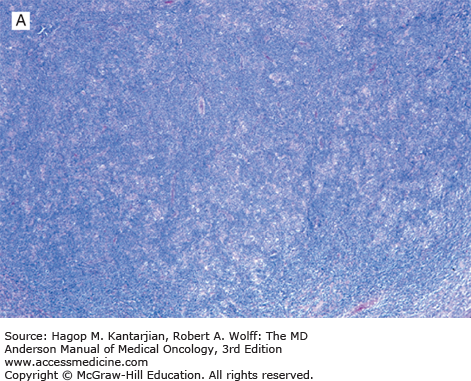
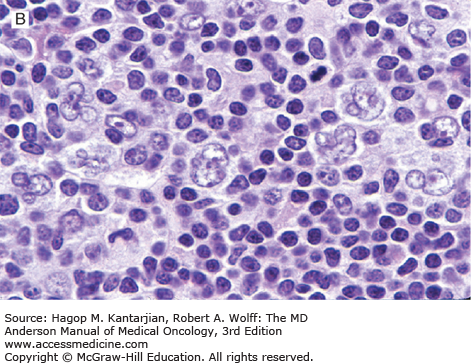
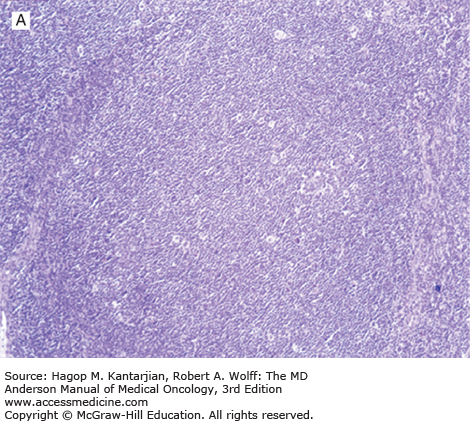
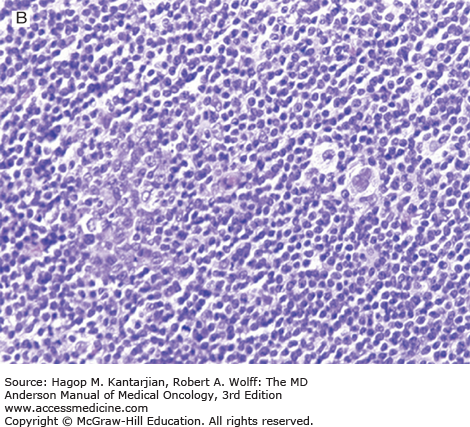
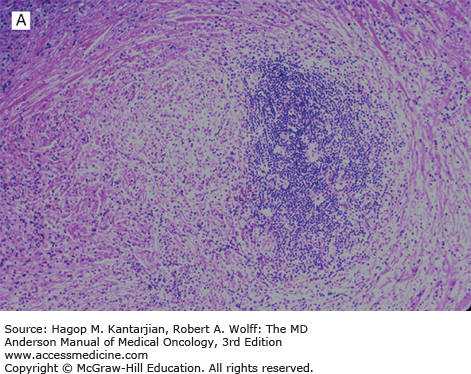
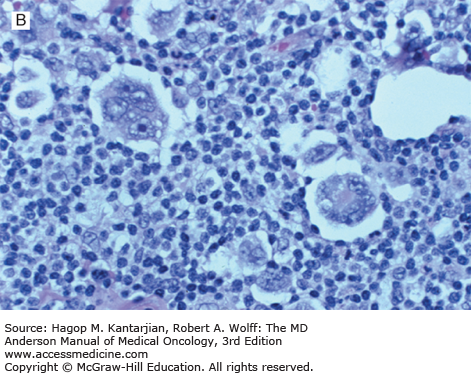
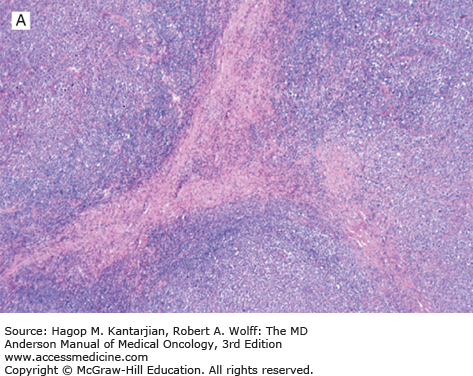
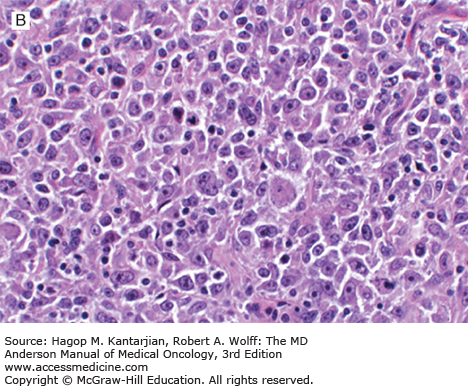
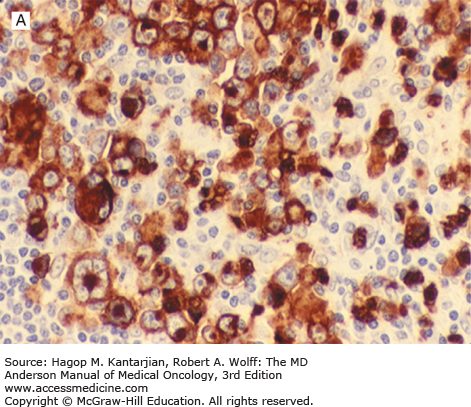
Nodular lymphocyte-predominant Hodgkin lymphoma is characterized by effacement of nodal architecture by variably sized, vague nodules composed of numerous small lymphocytes, histiocytes, and characteristic neoplastic LP cells (Fig. 10-1A) (1,8). These cells are typically large, with pale cytoplasm and polyploid vesicular nuclei containing inconspicuous nucleoli resembling kernels of popped corn, hence the nickname popcorn cells (Fig. 10-1B). However, LP cells can exhibit a range of cytologic appearances. These cells can be round and/or have relatively prominent nucleoli. Eosinophils, neutrophils, and plasma cells are usually absent in NLPHL, and there is no associated necrosis or fibrosis.
FIGURE 10-1
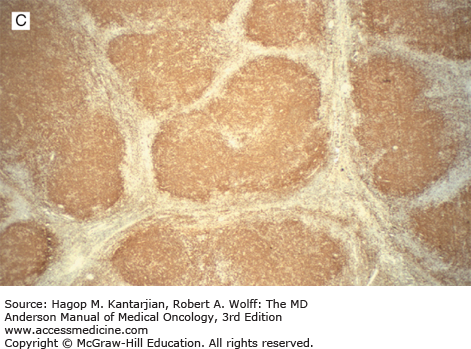
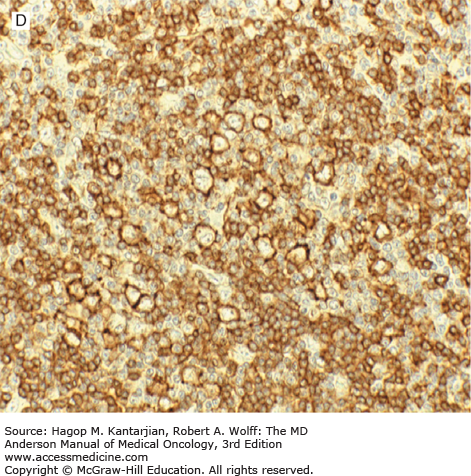
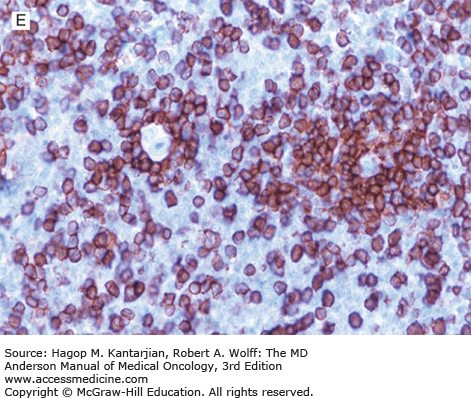
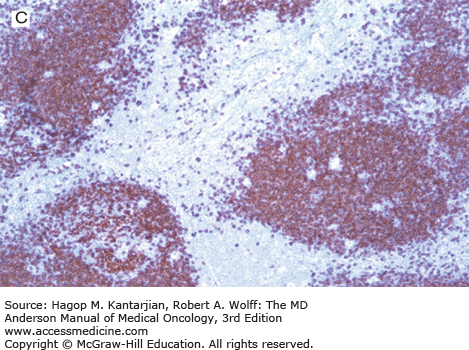
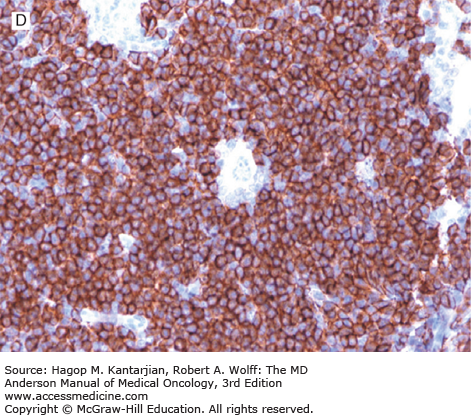
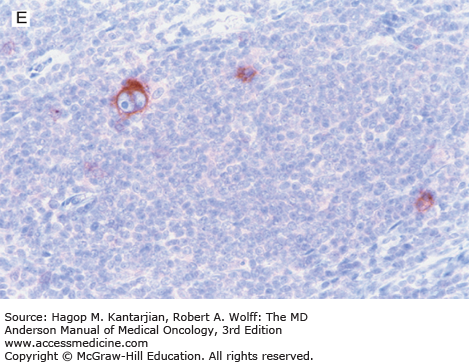
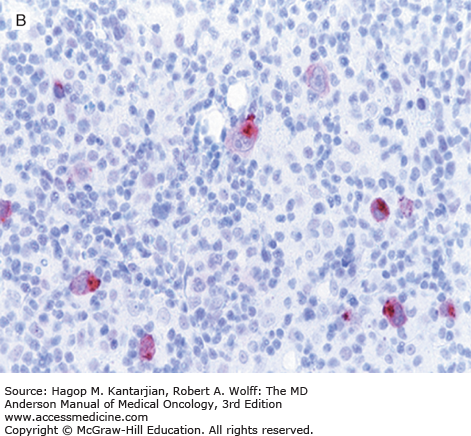
Nodular lymphocyte-predominant Hodgkin lymphoma. A. At low-power magnification, the neoplasm is vaguely nodular. B. At high-power magnification, large lymphocytic and histiocytic (LH) cells, resembling popped kernels of corn, are identified in a background of reactive lymphocytes and histiocytes. C and D. Immunohistochemical stain for CD20. C. At low-power magnification, this immunostain highlights the nodular pattern and shows numerous B cells in the nodules. D. At high-power magnification, large LH cells and small reactive B cells are positive for CD20. E. Immunohistochemical stain for CD3. Scattered small reactive T cells are present and focally surround the LH cells (so-called rosetting).
Cases of NLPHL can also have diffuse areas. When diffuse areas are large, their presence often correlates with more aggressive disease. To reflect this change in clinical behavior, many pathologists diagnose such cases as NLPHL with progression to T-cell/histiocyte-rich large B-cell lymphoma, also described as T-cell–rich B-cell lymphoma (TCRBCL). Other pathologists use the term NLPHL with large diffuse areas and suggest that the diffuse areas may represent the beginning stages of progression to DLBCL. The boundary between NLPHL with diffuse areas and TCRBCL remains blurred. Most cases previously designated as diffuse LPHL, as defined previously (9), are now classified differently. With appropriate workup, these cases are usually classified as NLPHL with large diffuse areas, lymphocyte-rich cHL, or TCRBCL.
Gene expression profiling of NLPHL to determine the origin and pathogenesis of LP cells found significant similarities between NLPHL, TCRBCL, and cHL (10). Overall, LP cells are thought to derive from antigen-selected GC B cells (5). LP cells also demonstrate deregulation of numerous apoptosis regulators and putative oncogenes and a partial loss of their B-cell phenotype. In addition, there is constitutive activation of nuclear factor-κB (NF-κB), Janus kinases/signal transducers and activator of transcription (JAK/STAT) pathway, and aberrant extracellular-regulated kinase signaling.
Nodular lymphocyte-predominant Hodgkin lymphoma is immunophenotypically distinct from other types of HL. The LP cells usually express leukocyte common antigen (LCA; CD45), immunoglobulin J chain, B-cell antigens (CD19, CD20, CD22, CD79A, and BCL-6), and epithelial membrane antigen (EMA) and are negative for CD15 and CD30 (Figs. 10-1C and D). These results suggest that the LP cells are B cells that arise from the GC. The LP cells are negative for T-cell antigens but are often surrounded by a rosette of small, reactive T cells that may be positive for pan-T-cell antigens and CD57 (Fig. 10-1E). Epstein-Barr virus is almost always absent in the LP cells of NLPHL.
Lymphocyte-rich cHLs (LRHLs) have been recognized that resemble NLPHL histologically but are cHL immunophenotypically (1,6,8). The frequency of this type of HL is not well known but is most likely low (<5%). Clinically, patients with LRHL are similar to patients with other subtypes of cHL or have clinical findings intermediate between NLPHL and cHL. Unlike patients with NLPHL, late relapse is uncommon in patients with LRHL.
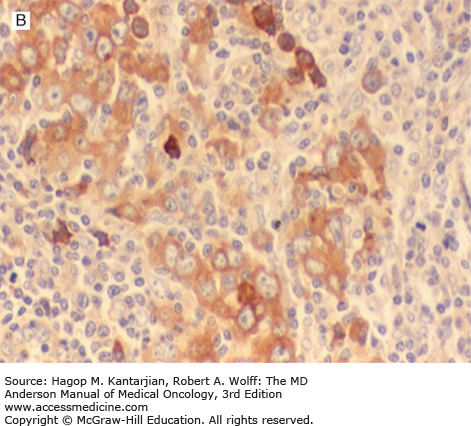
Histologically, these tumors are rich in small lymphocytes and histiocytes. Granulocytes and plasma cells are usually infrequent. Necrosis is usually not present. Lymphocyte-rich cHL may be either nodular or diffuse. The nodular type closely resembles NLPHL. Vague nodules of numerous small lymphocytes are present, and the nodules may have a small compressed GC (Figs. 10-2A and B). The neoplastic cells are present in the mantle zones of the nodules. These neoplastic cells usually resemble Reed-Sternberg and typical mononuclear variant cells (so-called Hodgkin cells) rather than LP cells. The cell composition is similar in diffuse cases of LRHL, but nodularity is minimal or absent. Immunohistochemical studies of LRHL show that the large neoplastic cells have an immunophenotype similar to that of all cHL cases, positive for CD15 and CD30 and negative for LCA (CD45) (Figs. 10-2C,D,E).
FIGURE 10-2
Lymphocyte-rich classical Hodgkin lymphoma, nodular variant. A. At low-power magnification, the neoplasm has a nodular pattern and is rich in reactive small lymphocytes (resembling nodular lymphocyte-predominant Hodgkin lymphoma). B. At high-power magnification, large neoplastic cells (so-called Hodgkin cells) are identified in the mantle zone of the follicle (note reactive germinal center to left of field). C and D. Immunohistochemical stain for CD20. C. The nodules contain numerous small reactive B cells. D. The Hodgkin cells are negative for CD20. E. Immunohistochemical stain for CD30. The Hodgkin cells are positive.
In a study of NLPHL by the European Task Force on Lymphoma (8), a large number of tumors that had been classified as NLPHL were reviewed; the diagnosis was confirmed in only half of these cases. Most of those excluded were reclassified as LRHL.
Nodular sclerosis (NS) is the most common form of cHL, representing 60% to 70% of all cases in Western countries; it is also the most common type of cHL in patients younger than age 50 years. Whites are affected more often than others, and nodular sclerosis HL (NSHL) is much less frequent in Asian countries (2,3). The age-adjusted incidence rate of NSHL has been stable since 1993 to 2008 in the United States according to the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) data (2). The male-to-female ratio is approximately equal. Nodular sclerosis HL has a marked predilection for involving mediastinal, supraclavicular, and cervical lymph nodes. A mediastinal mass is very common, and the thymus may be involved.
Nodular sclerosis HL is characterized by a triad of findings: (1) a nodular pattern, (2) broad bands of fibrosis that outline the nodules, and (3) characteristic mononuclear cell variants known as lacunar cells (Fig. 10-3). A lacunar cell has abundant clear cytoplasm with a sharply demarcated cell membrane. In formalin-fixed tissue, a characteristic artifact occurs. The cell cytoplasm retracts, leaving a clear space or lacuna surrounding the cell, hence the origin of the name. The typical lacunar cell has a polylobulated nucleus with one or multiple small nucleoli. However, lacunar cells can show great morphologic variability and can be round with prominent nucleoli, or they may resemble large noncleaved cells. A heterogeneous mixture of reactive cells may be seen in HL, including small lymphocytes, histiocytes, eosinophils, neutrophils, and plasma cells in variable numbers.
Nodular sclerosis HL has been graded (as 1 or 2) by the British National Lymphoma Investigation (BNLI) group (11) according to the numbers of neoplastic cells and reactive cells present, and this grading system has been adopted by the WHO classification (1). Grade 2 cases of NSHL show numerous neoplastic (lacunar) cells and depletion of reactive lymphocytes. Lymphocyte-depleted and syncytial variants (Fig. 10-4) of NSHL have been described; these cases are the outermost examples of grade 2 NSHL. The syncytial variant of NSHL is composed of sheets of neoplastic cells and necrosis.
The mixed cellularity variant of HL (MCHL), the second most common type, affects 15% to 25% of all patients with the disease and is the most common form in patients older than age 50 years (1,12). Males are affected more often than females. According to NCI SEER data, MCHL is relatively more common in African Americans and Hispanic Americans than in whites in the United States. A substantial percentage of patients with MCHL have clinical stage III or stage IV disease and B symptoms.
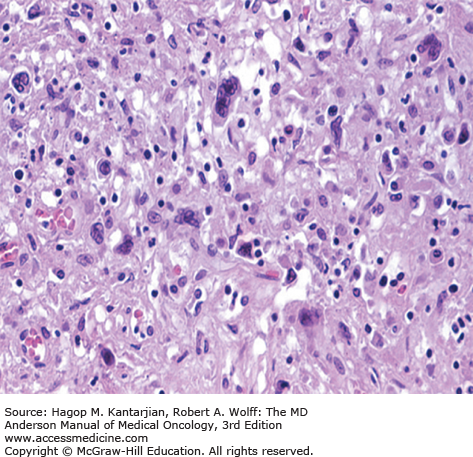
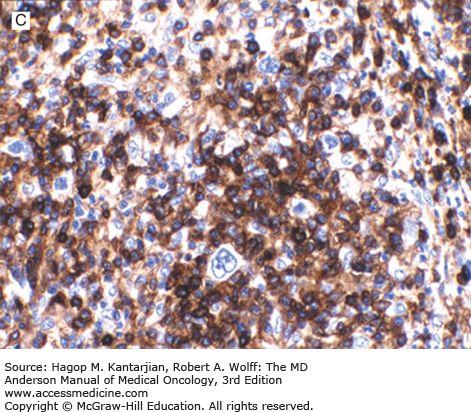
Mixed cellularity HL is characterized by a large number of Reed-Sternberg cells and Hodgkin cells in a background of numerous eosinophils, plasma cells, histiocytes, and granulocytes in varying proportions (1) (Fig. 10-5). The lymph node architecture is usually diffusely replaced. Partially involved lymph nodes show selective paracortical infiltration. Disorderly fibrosis may be seen, but the broad fibrous bands and capsular fibrosis characteristic of NSHL are absent.
FIGURE 10-5
Mixed cellularity Hodgkin lymphoma. A. Classic Reed-Sternberg cell (center of field) and mononuclear Hodgkin cells can be appreciated in a background of reactive lymphocytes, histiocytes, and eosinophils. B. Immunostain for Epstein-Barr virus latent membrane protein type 1. The neoplastic cells are positive.
Two variants of MCHL can be relatively more difficult to diagnose. In the interfollicular variant, which most likely represents partial involvement of lymph nodes by HL, the tumor is located in the interfollicular region and is often associated with reactive follicular hyperplasia and marked plasmacytosis. In the epithelioid histiocyte-rich variant, numerous epithelioid histiocytes and granulomas are present; these can be so numerous as to obscure the neoplastic cells. The importance of these variants of MCHL lies in their unusual histologic findings.
Lymphocyte-depleted HL (LDHL) is the least common type, representing 1% of all cases (12). In the NCI SEER study, it was shown that the age-adjusted incidence rate for LDHL has decreased. This decrease is most likely explained by the recognition by pathologists that many tumors previously classified as LDHL are, in fact, non-HLs (such as anaplastic large-cell lymphoma). Improved classification is the result of application of immunohistochemical and molecular methods to the study of these tumors.
Patients with LDHL are usually elderly, and LDHL is rare in individuals younger than 40 years old. There is a slight male predominance. Whites and African Americans are equally affected. Most patients have advanced clinical stage and B symptoms. Patients commonly have a large contiguous mass of matted lymph nodes or diffuse visceral involvement. The diffuse fibrosis type of LDHL commonly has a subdiaphragmatic distribution. Lymphocyte-depleted HL has the poorest prognosis of all types of HL (12).
The LDHL category includes two variants originally recognized by Lukes and Butler: diffuse fibrosis and reticular (Fig. 10-6). The diffuse fibrosis variant of LDHL is characterized by an extensive proliferation of disordered, hypocellular fibrosis. Diagnostic HRS cells can be difficult to find and may be spindled within dense collagen. Reactive inflammatory cells are relatively few. The reticular variant of LDHL has numerous HRS cells and bizarre variants that have been termed pleomorphic variants. These cells may exhibit marked variations in nuclear number and shape, often with giant nucleoli. Normal small lymphocytes are infrequent compared with the other subtypes of HL. Necrosis is common and may be extensive. The HRS cells and pleomorphic variants may be found in sheets. Mitotic figures are usually numerous.
Overall the mature B-cell origin of the HRS cells is not readily apparent because HRS cells have a very unusual phenotype and have expression of genes that are seen on many hematopoietic cell types. The neoplastic cells are positive for CD15 and CD30 and negative for LCA (CD45) (Fig. 10-7) and EMA (1). B-cell antigens, such as CD20, CD79A, PAX-5/BSAP, and MUM1/IRF4, are expressed in a subset of cases. CD20 expression is often weak. T-cell antigens are usually not expressed by the neoplastic cells. BCL-2 is positive in up to half the cases and has been correlated with poorer prognosis (13). Epstein-Barr virus is relatively common in cHL, but its frequency varies greatly among different types.
HODGKIN LYMPHOMA: STAGING AND THERAPY
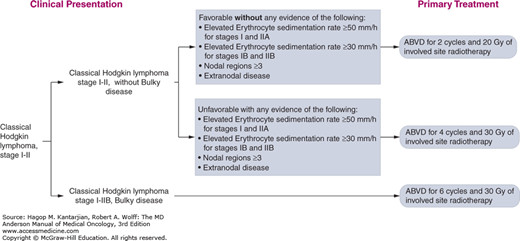
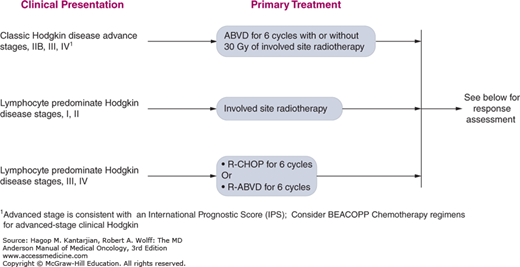
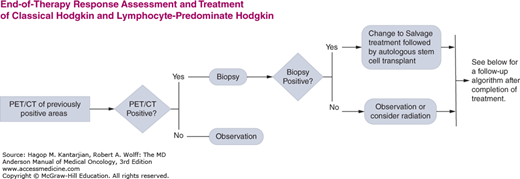
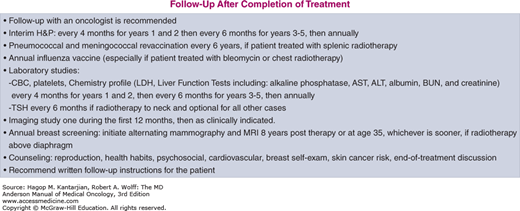
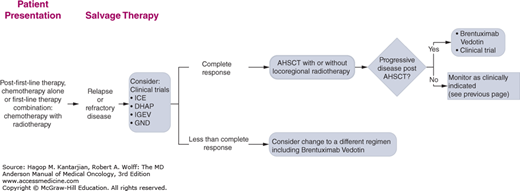
The University of Texas MD Anderson Cancer Center (MDACC) diagnostic and treatment algorithms for HL are shown in Fig. 10-8.
FIGURE 10-8
A-I. MD Anderson Cancer Center algorithms for Hodgkin lymphoma. ABVD: Doxorubicin, bleomycin, vinblastine, dacarbazine; R-CHOP: Rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-ABVD: Rituximab, Doxorubicin, bleomycin, vinblastine, dacarbazine; ICE: Ifosfamide, Carboplatin, and Etoposide; DHAP: High dose cytarabine, cisplatin and dexamethasone; IGEV: Ifosfamide, gemcitabine, vinorelbine and prednisone; GND: Gemcitabine, Navelbine and Doxil. © 2014 The University of Texas MD Anderson Cancer Center.
The Ann Arbor system for staging patients with HL at the time of initial presentation forms the basis for the treatment of disease and has allowed comparison of results achieved by different investigators for more than two decades. Important modifications of the Ann Arbor system were developed at the Cotswold Conference in 1989 (Table 10-3) (14). Since then, the staging evaluation has been changing. A recent recommendation for the staging procedure in lymphoma described the importance of positron emission tomography (PET)–computed tomography (CT) scan for fluorodeoxyglucose (FDG)-avid lymphomas, of which HL is one example (15). For HL and other FDG-avid lymphomas, PET-CT improves the accuracy of staging compared to CT scans for nodal and extranodal sites (16). Positron emission tomography–CT leads to change in stage in 10% to 30% of patients, more often upstaging, although alteration in treatment occurs in fewer patients with no demonstrated impact on overall outcome. However, PET-CT is critical as a baseline measurement before therapy to increase the accuracy of subsequent response assessment (17). In addition, contrast-enhanced CT scan should be included for a more accurate measurement of nodal size if required for clinical trials.
| Stage I | Involvement of a single lymph node region or lymphoid structure (eg, spleen, thymus, Waldeyer ring) or a single extralymphatic site. |
| Stage II | Involvement of two or more lymph node regions on the same side of the diaphragm; localized contiguous involvement of only one extralymphatic organ or site and lymph node region on the same side of the diaphragm (IIE). The number of anatomic regions involved should be indicated by a subscript (eg, II3). |
| Stage III | Involvement of lymph node regions on both sides of the diaphragm (III), which may also be accompanied by spleen involvement (IIIS) or by localized contiguous involvement of only one extralymphatic organ or site (IIIE) or both (IIISE). |
| Stage IV | Diffuse or disseminated involvement of one or more extranodal organs or tissues, with or without associated lymph node involvement. |
| Modifying Features | |
| A | No symptoms |
| B | Fever (>38°C), drenching night sweats, unexplained weight loss of >10% body weight within the preceding 6 months |
| X | Bulky disease: greater than one-third widening of the mediastinum, >10 cm maximum diameter of a nodal mass |
| E | Involvement of a single extranodal site that is contiguous or proximal to the known nodal site |
| CS | Clinical stage |
| PS | Pathologic stage (as determined by laparotomy) |
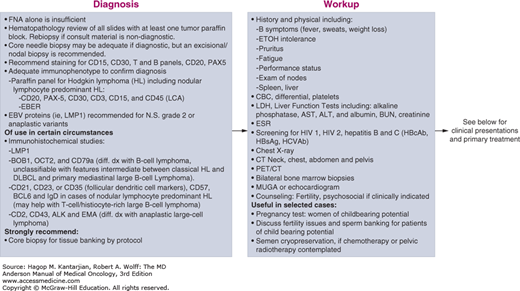
The initial evaluation of patients with HL has both prognostic and therapeutic significance (see Fig. 10-8). Routine studies that should be performed include a complete blood cell count with differential, electrolytes, blood urea nitrogen (BUN), creatinine, liver function tests, lactate dehydrogenase (LDH), albumin, pregnancy test in women of childbearing age, erythrocyte sedimentation rate (ESR), pulmonary function test (PFT) with carbon monoxide diffusing capacity (DLCO), evaluation of cardiac ejection fraction, chest x-ray, CT of neck, chest, abdomen, and pelvis, and PET-CT (Table 10-4).
| History and examination | Identification of B symptoms |
| Radiologic and other assessments | Chest radiograph |
| Computed tomographic (CT) scans including neck, chest, abdomen, and pelvis whole-body positron emission tomography (PET) scan | |
| Echocardiogram or multigated acquisition (MUGA) scan | |
| Pulmonary function tests | |
| Human immunodeficiency virus serology | |
| Pregnancy test in women of childbearing age | |
| Hematologic procedures | Complete blood count with differential |
| Erythrocyte sedimentation rate (ESR) | |
| Bilateral bone marrow aspiration and biopsy | |
| Biochemical procedures | Liver function tests |
| Serum albumin | |
| Lactate dehydrogenase | |
| Procedures for use under special circumstances | Ultrasound scanning |
| Magnetic resonance imaging |
Bone marrow biopsy has been standard in lymphoma staging. However, the high sensitivity of PET-CT for bone marrow involvement has recently led to questioning the use of bone marrow biopsy as a staging procedure in several common histologies, including HL (15). In one study in HL, 18% of patients had focal skeletal lesion on PET-CT, but only 6% had positive bone marrow biopsy, all with advanced-stage disease on PET-CT (18). Patients with early-stage disease rarely have marrow involvement in the absence of a suggestive PET finding, and those with advanced-stage disease rarely have marrow involvement in the absence of disease-related symptoms. Although the issue is controversial and some institutions still perform bone marrow biopsy for the initial staging evaluation, almost all patients would not have been allocated to another treatment based on bone marrow biopsy results. Thus, the recommendation states that bone marrow biopsy is no longer indicated for the routine staging of HL.
Magnetic resonance imaging (MRI) has not superseded CT scanning of the chest and abdomen in the evaluation of HL. It is largely restricted to the assessment of specific situations such as bony involvement and spinal cord compression as well as in lieu of CT scans in pregnant patients.
In HL patients at a low clinical stage (CS)—that is, CS I or CS II—several prognostic factors, based largely on patients treated only with radiotherapy, have been identified through retrospective studies. Adverse factors are: (1) advanced age, which correlates with the presence of occult abdominal disease and with poor results of salvage therapy; (2) male sex; (3) MC histologic type, which is associated with the presence of occult abdominal disease; (4) B symptoms, also associated with the presence of occult abdominal disease; (5) large mediastinal mass, defined as a mass measuring greater than one-third the chest diameter on a standard chest radiograph; (6) a larger number of involved nodal regions; (7) an elevated ESR; (8) anemia; and (9) a low serum albumin level (19,20).
International organizations have defined various systems that calculate the risk of recurrence of disease or, in some cases, death, after treatment for HL. The European Organization for the Research and Treatment of Cancer (EORTC) has defined CS I and CS II patients as having an unfavorable risk of development of recurrence if any of the following factors apply: (1) age >50 years, (2) no symptoms present with ESR >50 mm/h or B symptoms with ESR >30 mm/h, (3) large mediastinal mass, (4) stage II, or (5) at least four nodal regions involved (21). The GHSG has assigned CS I and CS II patients to the category of unfavorable disease with any of the following adverse factors: (1) large mediastinal mass, (2) at least three nodal regions involved, (3) no symptoms present with ESR >50 mm/h or B symptoms with ESR >30 mm/h, or (4) localized extranodal infiltration (so-called E lesions) (Table 10-5) (22). In advanced disease, the International Prognostic Score (IPS) was developed on the basis of an analysis of 5,141 patients most of whom were initially treated with an anthracycline-containing chemotherapy regimen. Seven factors were identified, as shown in Table 10-6 (23).
EORTC Unfavorable (presence of any of the following): Age ≤50 years ESR >50 mm/h without B symptoms, ESR >30 mm/h with B symptoms ≤4 nodal sites of involvement Bulky mediastinal mass |
GHSG Unfavorable (presence of any of the following): ESR >50 mm/h without B symptoms, ESR >30 mm/h with B symptoms ≤3 nodal sites of involvement Bulky mediastinal mass Extranodal involvement |
Prior to 1999, the criteria used to assess response to therapy were not routinely standardized. The International Working Group (IWG) formulated guidelines for the assessment of response to therapy in 1999 (24). The criteria were based on CT scan and have been used internationally. With the introduction of the PET scan, the guideline has been updated twice, in 2007 and in 2014 (15,25). Based on the high negative predictive value (95%-100%) and positive predictive value (>90%) of PET scan in the response evaluation for HL (26), current recommendations for response evaluation clearly state that PET-CT is more accurate than CT for end-of-treatment assessment. Previous guidelines for reviewing PET scan were based on imprecise visual interpretation, whether the scan is positive or negative, and whether it is intended for end-of-treatment evaluation using mediastinal blood pool as the comparator (27). More recent guidelines recommend using a 5-point scale assessment (Deauville criteria, Table 10-7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree