EPIDEMIOLOGY
Through 2006, there were 982,498 cases of AIDS reported to the Centers for Disease Control (CDC) in the United States
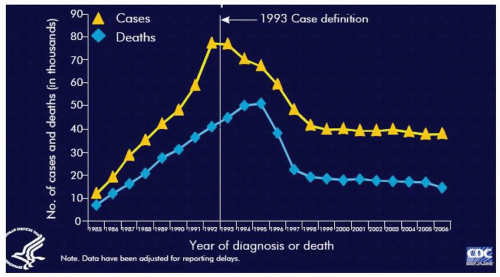
(Table 9.1). AIDS cases rapidly increased in the U.S. during the 1980s and peaked in 1992. In the initial two decades (1981 to 2001) of the HIV epidemic, an estimated 1.3 to 1.4 million persons in the United States were infected with HIV and 816,149 cases of AIDS and 467,910 AIDS-related deaths were reported to the CDC. Following introduction of combination antiretroviral therapy in the late 1990s, the number of new AIDS cases and deaths declined dramatically and have remained stable since, at approximately 38,000 and 16,000, respectively
(Table 9.2). Through 2006, there have been 545,805 deaths secondary to AIDS in the United States.
An estimated 1.2 million people in the United States are living with HIV infection with the adult HIV prevalence estimated to be 0.6%. Approximately 25% of these HIV-infected people are unaware they are infected. Nearly three quarters of people in the United States living with HIV infection are males. However, women have become increasingly affected by the epidemic; by 2001 to 2004 women made up 27% of AIDS cases compared with 15% in 1981 to 1985. In the United States, more than one quarter of all new HIV infections and AIDS diagnoses are in women. Approximately one third of people living with HIV or AIDS in the United States are women.
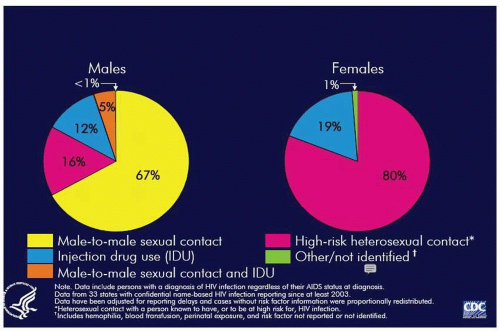
Among HIV-infected persons in the United States, men having sex with men (MSM) remains the largest single group, accounting for 45% of cases. Heterosexual transmission accounts for 27% and persons infected via injection drug use account for 22%. Among women nearly 80% of cases are associated with high-risk heterosexual contact
(Fig. 9.1). In the United States, over 80% of the cumulative cases of AIDS in women have occurred among persons in the reproductive age group. Among the pediatric cases of AIDS, over 90% were secondary to maternal-infant transmission.
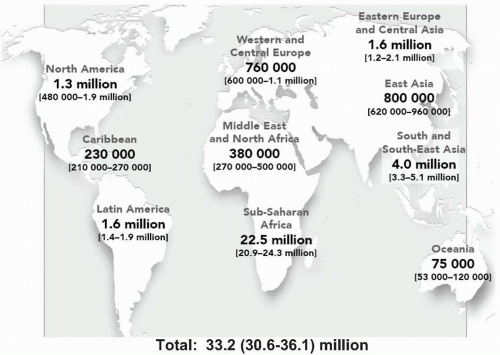
It is important to recognize that the HIV-AIDS epidemic in the United States exists in the context of a much larger and more devastating global pandemic of HIV infection and AIDS with cases reported to the World Health Organization from virtually every country. The Joint United Nations Program on HIV/AIDS (UNAIDS) estimates that more than 65 million adults and children worldwide have been infected with HIV. Approximately 25 million of these persons have died from AIDS and >40 million are currently living with HIV infection. Of these HIV-infected persons, over 95% live in developing countries
(Table 9.3, Fig. 9.2). UNAIDS estimates that there are 5 million persons (4.7 million adults and 620,000 children) newly infected with HIV each year. In addition, 3 million deaths per year occur as the result of the complications of AIDS. Of these, almost all live in developing countries.
The geographic distribution of HIV infection varies considerably (
Fig. 9.2). Sub-Saharan Africa, with 24.5 million people affected by HIV, accounts for 63% of worldwide HIV infections.
In the developing world, most adult transmission occurs as the result of heterosexual sex (approximately 85%). Parenteral transmission by sharing unsterilized needles and syringes among injection drug users (IDUs) is the second most common mode of transmission; this is particularly prominent in Central and Southeast Asia, Eastern Europe, and South America. Outside of the sub-Saharan Africa, one-third of HIV infections are acquired through injection drug use. Mother-to-infant (perinatal) transmission is third; this mode is common in sub-Saharan Africa, where it is responsible for 15% to 20% of HIV infections. In other regions of the developing world, perinatal transmission accounts for 5% to 10% of HIV infections. Other routes of transmission in developing countries include blood transfusion (<10%) and medical injection with contaminated needles (<5%).
In the new millennium, women are bearing an increasing burden of the HIV pandemic. This in turn has important implications for mother-to-child transmission of HIV. It is estimated that women make up 42% of HIV-infected persons worldwide, and 70% of HIV-infected women live in sub-Saharan Africa. A quarter of new HIV infections occur in adolescents and young adults aged <25 years and HIV infection rates are three to six times higher in female adolescents than male adolescents.
During the past 15 years, we have seen the emergence of parallel HIV-AIDS epidemics. Recent advances in our understanding of the natural history of the immunodeficiency associated with HIV infection and the development of highly active anti-retroviral therapy (HAART) have led to dramatic decreases in mortality and morbidity in persons with access to diagnostic monitoring (viral load, CD4 counts) and HAART
(Fig. 9.3). In the industrialized countries, management has changed from a focus on treating opportunistic infections (OIs) and caring for the dying to focus on compliance and quality of life. HIV has become an incurable chronic disease (in the United States and other developed countries), with HIV-infected persons living longer and productive lives. However, they are at increased risk for a variety of disorders and complications associated with HIV infection and anti-retroviral therapy
(Box 9.1). Unfortunately, in both the developing world and disadvantaged
communities in industrialized countries, the HIV epidemic continues to weave its pattern of mortality and morbidity.
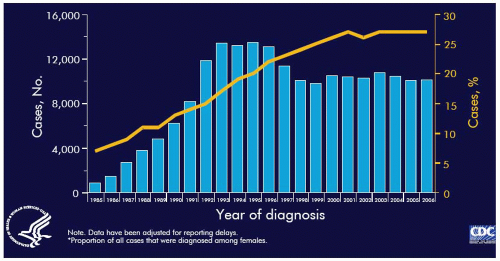
Although overall, women represent only 18% of all cumulative reported AIDS patients through 2006, the number of AIDS cases in women has been steadily increasing each year. The increasing presence of women in the AIDS epidemic in the United States is clearly demonstrated by comparing the prevalence of women among the first 100,000 reported cases of AIDS (9%) and 2006 (26.6%). Not only do women account for an increasing proportion of reported cases of AIDS but AIDS has been among the leading causes of death in reproductive age women in the United States since 1992.
Globally, a similar pattern has emerged; whereas more men have been infected with HIV than women (except in sub-Saharan Africa), new infections are increasing more rapidly among women. These gender differences are explained by a variety of biologic, behavioral, and social factors. These include (i) HIV is more easily transmitted from men to women; (ii) young women and girls are more susceptible to infection because of a greater degree of cervico-vaginal fragility than is seen in older women; (iii) women are more likely than men to
have asymptomatic STDs, which are more likely to go untreated resulting in an increased risk for acquisition of HIV; and (iv) social factors in which women lack control over conditions under which they have sex (e.g., lack of condom use). The HIV-AIDS epidemic has had its greatest impact on minority populations in the United States, particularly among African-Americans and Hispanics. AIDS rates among these groups are three to four times greater than those among whites. Among women this racial/ethnic disparity is strikingly magnified.
This rising rate of HIV infection among women mandates that providers of healthcare to women in the United States play an increasing role in the effort to meet the challenge of the AIDS epidemic. Specifically, providers of healthcare to women will need to provide care to not only HIV-infected pregnant women but also HIV-infected nonpregnant women and to become actively involved in the efforts to control the spread of HIV. In addition, obstetrician-gynecologists must be able to provide appropriate counseling and medical care for these women.
The current picture of the U.S. HIV-AIDS epidemic is very different from that seen when the epidemic initially emerged in the 1980s. The race and gender of the HIV-infected population is increasingly minority (African-American and Hispanic), increasingly female, and increasingly in the reproductive age group. In
Table 9.2, the number of cases reported each year according to sex, race or ethnic group, and category of exposure are depicted. MSM (including IDUs) make up the largest group each year but are decreasing as a proportion. The groups with the largest percentage increases over time were the heterosexual group and the group without risk factors reported or identified. Many of the latter especially among females are ultimately reclassed as heterosexual. In addition,
Table 9.2 describes the increasing percentage of women, members of racial or ethnic minorities, and AIDS patients infected through heterosexual contact. Introduction of new, more effective antiretroviral agents that will result in longer, healthier lives for HIV-infected persons plus the new demographic profile of the epidemic suggests HIV infection among pregnant and nonpregnant women will continue to be a challenge for women’s healthcare providers.
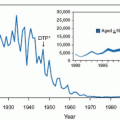
The most dramatic favorable trend of the AIDS epidemic in the United States and Western and Central Europe has been the dramatic reduction in mother-to-infant transmission (MTIT) of HIV
(Fig. 9.4). From a peak of 1,700 in 1992, the estimated number of children with perinatally acquired AIDS has declined over 75%. This tremendous accomplishment is attributed to several factors as noted in
Box 9.2.
ETIOLOGY AND PATHOGENESIS
AIDS is caused by infection with HIV. Two types of HIV that are able to cause AIDS in humans have been identified: HIV-1 and HIV-2. HIV-1 is substantially more common with HIV-2 limited to West Africa. The major routes for infection with HIV are by blood/blood products, intimate sexual contact, and mother-to-child transmission (antepartum, intrapartum, or postpartum through breastfeeding).
HIV based on its characteristic dense, cylindrical, nucleoidcontaining core proteins, genomic RNA, and the reverse transcriptase enzyme, is classified as a member of the retrovirus family. All members of the retrovirus family code for the reverse transcriptase enzyme (RNA-dependent DNA polymerase), which allows the viral RNA to be transcribed into a DNA copy. It then integrates into the genome of the infected cell and replicates via the proviral DNA.
HIV is a member of the lentivirus subfamily of human retroviruses. Lentiviruses characteristically result in chronic indolent infections with involvement of the nervous system, long periods of clinical latency, and weak humoral immune responses complicated by persistent viremia.
As determined by their genetic make-up, HIV-1 viruses have been divided into three groups: M, N, and O. HIV-1 has further diversified into nine subtypes. In addition, many circulating recombinant forms encoding genetic structures from two or more of these subtypes have evolved. It is this ongoing evolution of HIV-1 diversity that has led to difficulties developing preventive vaccines and resistance to treatment.
Subtype C viruses dominate and account for 55% to 60% of HIV infections worldwide. In the United States, Western Europe, Oceania, Latin America, and the Caribbean, subtype B predominates. In sub-Saharan Africa, subtypes A, D, F, G, C, H, J, K and CRF occur, whereas in south and southeast Asia subtypes B and AE and in east Asia subtypes B, C, and BC predominate.
HIV-1 Virion and Genomic Structure
The continued pandemic spread of HIV-1 nearly 30 years after first emerging, strongly suggests that HIV-1 can counteract the human innate, adaptive, and intrinsic immunity. Although HIV-1 is of modest genomic size (<10 Kb) and contains only a few genes, it is adept at simultaneously utilizing host cell mechanisms and neutralizing and evading the host immune system.
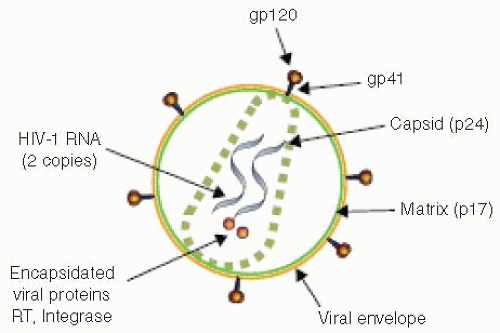
The structure of HIV is schematically depicted in
Figure 9.5. The HIV virion contains a lipid bilayer envelope that surrounds a cone-shaped core composed of the viral p24 protein capsid that contains two identical RNA strands, reverse transcriptase (RT), integrase, and the nucleocapsid proteins p9 and p6. The inner portion of the viral membrane is surrounded by the p17 core (Gag) protein that provides the matrix (MA) for the viral structure and is vital to the virion’s integrity. The viral surface is made up of 72 knobs of the envelope glycoproteins including the gp 120 external surface envelope protein and a gp 41 transmembrane protein. The envelope glycoprotein is responsible for initial interactions between HIV and host cells. This includes binding and entry into host cells, membrane fusion and syncytium production. The gp 120 glycoprotein is divided into distinct domains; those with little variation in amino acid sequences are called constant (C) regions whereas those with substantial variation are called variable (V) regions. The V3 loop, a part of variable region V3, is the major epitome that elicits neutralizing antibody. Variation in the amino acid sequence of the V3 loop has been associated with viral tropism for specific cell types such as lymphocytes, monocytes, or CNS cells. In addition, the lipid bilayer is studded with host proteins, including class I and II major histocompatability complex (MHC) antigens.
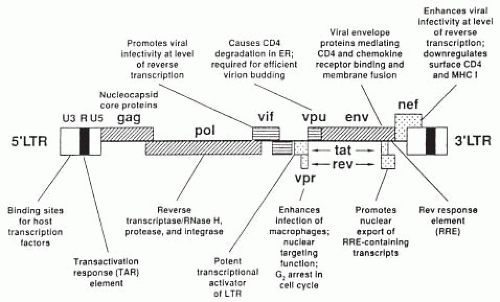
Based on its genetic complexity, HIV is unique among the retroviruses
(Fig. 9.6). In addition to the three structural (essential) genes,
gag, pol, and
env, which encode the core proteins, reverse transcriptase enzyme and envelope proteins, HIV contains an elaborate set of additional (regulatory) genes that determine whether or not virus is made and if so, control the level of virus production. These genes function to regulate production of viral proteins. To date, six additional genes have been identified in the HIV genome. As noted in
Figure 9.6, these nine genes are arranged along the viral DNA and flanked by long-terminal repeats (LTRs), which do not code for any protein but rather initiate expression of other viral genes. The identified functions for these genes are listed in
Table 9.4. It is the distinct, but concerted actions of these 9 genes that underlie the profound pathogenicity of HIV-1.
The
gag-derived proteins (
Table 9.4) comprise the structural components of the virus. The viral core contains proteins derived from the pol gene that are essential for viral replication. These include reverse transcriptase (RT), which synthesizes viral cDNA from viral RNA, integrase (IN), which catalyzes insertion of viral cDNA into the cellular DNA, and protease (PR), which cleaves the
gag and
pol precursor proteins to create the final form of the viral structural proteins during virion maturation. These enzymes are targets for antiretroviral therapy.
Several of these regulatory genes accelerate virus replication. The
trans-activator gene (
tat) is a positive feedback regulator that increases its own rate of synthesis and the synthesis of all viral proteins. Tat is essential for the replication of HIV-1. The
tat gene is responsible for the greatly increased replication of HIV seen in CD4+ T lymphocyte cells stimulated by an antigen. The auxiliary proteins Nef and Vif enhance the reverse transcription process. Nef is required during the viral production and assembly phase of the HIV life cycle but its effect is primarily apparent with HIV binding to the target cell and establishment of a provirus. The virion infectivity gene (
vif) increases the infectivity of the virus particle. As with Nef, Vif is required during viral production and assembly but its effect occurs during binding and provirus production.
Vif counteracts an intracellular antiviral activity that inhibits virus replication.
HIV-1 contains an unusual genetic switch, the regulator of virion protein expression (rev). The rev gene positively regulates expression of virion proteins whereas it negatively regulates expression of the regulatory genes. The Rev protein exerts its regulatory activity at a post-transcriptional level by inhibiting viral RNA splicing and by activating cytoplasmic transport of the unspliced and single spliced forms of HIV-1.
Vpr is a small auxiliary protein found in the preintegration complex that enhances HIV replication rate in macrophages. The Vpr protein plays a role during the late stages of virion morphogenesis by promoting the efficient release of the budding virions from the cell surface.
Viral Life Cycle
HIV-1 infects cells with the CD4 antigen, especially helper CD4+ T lymphocytes but also monocytes/macrophages, CNS cells and possibly placental cells. The CD4 molecule on the surface of these cells serves as the primary receptor for HIV-1, binding to the viral envelope glycoprotein gp 120. During primary infection, HIV infects cells of the macrophage lineage. Dendritic cells interact with mucosal T cells to participate in the initial establishment of infection.
Two additional cell surface molecules have been identified as cofactors for HIV cell entry and fusion. These coreceptors are the chemokine receptors CXCR4 and CCR5. This explains why certain strains of HIV-1 preferentially infect macrophages, whereas other strains preferentially infect CD4+T cells. The CXCR4 chemokine receptor is utilized by HIV-1 strains displaying T-cell line tropism and the CCR5 chemokine receptor is used by macrophage-tropic HIV-1 strains. Persons homozygous for a 32-nucleotide deletion in the CCR5 receptor have a high degree of resistance to sexually acquired HIV infection. With the heterozygous condition of CCR5, slow progression of HIV disease occurs. In addition, the homozygous deleted CCR5 genotype confers substantial protection from maternal-child vertical transmission.
In
Figure 9.7, the life cycle of HIV is outlined. The initial step in the life cycle of HIV begins when the virion binds to its future host cell. Following interactions of gp 120 with CD4 and one of the chemokine receptors (CCR5 or CXCR4), membrane fusion (mediated by gp 41) occurs. As a result HIV-1 enters into the host cell. Uncoating occurs, followed by reverse transcription of viral RNA and the production of double-stranded viral DNA. HIV-1 integrase then promotes insertion of this viral DNA into the host genome, resulting in HIV-1 provirus. The expression of HIV-1 genes is initially stimulated secondary to the action of host transcription factors with binding sites in the LTR. As a result of these enhancer-binding proteins, sequential production of various viral RNAs occurs. The first mRNAs produced encode for the Tat, Rev, and Nef regulatory proteins. Tat moves back to the nucleus where it up-regulates the activity of the LTR. Rev facilitates production of the viral structural proteins that allow assembly and morphogenesis of virions. Influenced by Nef, these HIV-1 virions bud from the host cell. Subsequent infection of other CD4+ cells reinitiates the HIV life cycle.
Disease Progression
HIV infection is characterized by substantial viral variability, genotypically, phenotypically, and clinically. Genetically, an infected individual may harbor heterogeneous HIV-1 genomes known as viral quasispecies. This heterogeneity results from the high level of viral replication that distinguishes HIV from most other human infectious agents. The half-life of an HIV virion is extremely short and as a result half the entire plasma HIV population is replaced in less than 30 minutes. Thus, it is estimated that more than one billion virions are produced per day. Such a vast number of replication cycles allow the virus ample opportunity to develop genetic diversity. This opportunity
is further enhanced because viral DNA polymerase is error prone and thus viral replication errors are introduced by the reverse transcriptase enzyme.
HIV isolates also have phenotypic diversity in vitro, which correlates with clinical status. Species with tropism for T lymphocytes and ability to induce syncytium formation (SI) are associated with advanced disease. In contradistinction, phenotypes with tropism for macrophages and that are nonsyncytial inducing (NSI) are associated with primary and asymptomatic infection.
Great diversity is also present in the clinical course and rate of disease progression with HIV infection. Prior to the availability of highly effective antiretroviral therapies the average time elapsed from initial infection with HIV to the development of AIDS was 10 to 11 years. However, in approximately 20% of HIV-infected individuals, this progression was accelerated and AIDS developed within 5 years. At the opposite end of the spectrum an even smaller group (<5%) were long term nonprogressors. Which of these groups HIV-infected individuals fall into is the result of complex interactions between HIV, host immunogenetic factors (e.g., chemokine receptor status), and the environment.
Viral replication is influenced by the level of immune activation. Many components of the immune system respond to infection with HIV and are associated with inhibition of viral replication. However, there are circumstances in which specific immune mechanisms actually enhance viral replication. Following infection with HIV, the host produces successful protective responses including generation of envelope-specific antibodies with neutralizing activity and HIV-specific antibodies with neutralizing activity and HIV-specific cytotoxic T cells to a variety of HIV proteins. Current opinion holds that components of the natural or non-antigen-specific immune system (e.g., secretion of cytokines with antiviral activity by activated CD8+ cells) are more important in inhibiting HIV replication.
Originally, it was believed that after the initial primary infection during which high levels of HIV replication were present, viral replication entered a long latent phase during which HIV-infected persons were asymptomatic. In turn, this ultimately was followed by accelerated viral replication and depleted CD4+ T cells, which were thought to precede development of AIDS. However, research in the mid-1990s led to the currently held concept that viral replication and turnover of infected T cells are rapid and continuous, even during the asymptomatic stage.
There is a steady state of HIV in the blood with billions of virus particles continuously being produced by newly infected cells and then rapidly cleared. Plasma HIV levels are high and remain so because many new immune cells are constantly being infected. Thus HIV infection is a dynamic process where there are continuous rounds of de novo infection, replication, and turnover.
In nearly all HIV-infected patients, HIV-1 RNA can be detected in the plasma, following acute infection. Early in primary, acute infection, there is rapid viral replication that is unopposed by an effective immune response and produces a plasma viremia of 106 to 107 HIV-1 RNA copies per milliliter. There is a concomitant precipitous drop in CD4+ T cells which, within a few weeks, are partially restored as anti-HIV-specific immune responses are elicited. This results from the hematopoietic system replacing depleted T cells as the immune system attempts to limit viral replication. Following this immune response, the viral load drops dramatically by 2 to 4 log copies per milliliter and reaches a nadir or “set point” approximately 3 to 4 months after the
onset of symptoms in primary HIV-1 infection. However, even in the face of this reduced viremia, there is continuous viral replication and CD4+ T cells depletion occurring during all stages of HIV infection.
Infectivity
Dendritic cells (tissue and circulatory) play an important role in the pathogenesis of HIV infection. The primary function of these dendritic cells is to capture, process and present antigens to T cells. At mucosal sites (and skin) primary HIV infection includes either the capture of virions by and/or infection of dendritic cells contained in the epithelium and subsequent migration of infected dendritic cells to regional draining lymph nodes. Studies of simian immunodeficiency virus (SIV) infection in nonhuman primate models demonstrated that vaginal transmission leads to infection of CD4+ T lymphocytes, macrophages, and dendritic cells in the lamina propria. These regional lymph nodes are the site where HIV infection becomes established. Initial replication occurs in the regional lymph nodes, but this primary amplification is limited in scale. Subsequently, migration of infected T-lymphocytes or HIV virions into the bloodstream leads to secondary amplification in the gastrointestinal tract, spleen and bone marrow. This secondary amplification results in massive infection of susceptible cells.
During primary HIV-1 infection a peak viremia of 106 to 107 copies per mL of plasma occurs. As a result of HIV-1-specific CD8+ responses, the level of viremia is reduced by one or two orders of magnitude to reach the viral set point, which is the level of viremia for the chronic phase of infection. Early after initial transmission of HIV, the viral population is fairly homogenous. However, viral quasispecies begin to occur that are resistant to host immune responses and antiretroviral agents. In turn these mutant HIV strains are archived in long-lived cells and persist in viral reservoirs.
Three principal means of HIV transmission have been demonstrated. HIV may be transmitted by blood or blood products, sexual contact (homosexual or heterosexual), and perinatally (mother-to-child). MTCT may occur in utero (transplacental), intrapartum, or via breastfeeding. With introduction of molecular techniques (e.g., PCR), quantitation of virus in the blood demonstrated an association between viral load, CD4+ cell count and disease stage. HIV viral load is highest during the initial acute infection, drops dramatically during the asymptomatic stage, and rises again as HIV-infection progresses to AIDS.
Transfusion of blood from an HIV-infected donor is a very efficient means of transmission; the risk depends on disease stage, viral load, and phenotype of the predominant HIV strain in the donor. Prior to the recognition that HIV was present in blood and blood products thousands of transfusion recipients and hemophiliacs were infected with HIV. Improved donor screening techniques by blood banks and treatment of blood products has virtually eliminated receipt of blood and/or blood products as a risk for HIV transmission in developed countries.
Among IDUs, shared needles are the responsible risk factor. The risk for HIV transmission among IDUs correlates directly with the incidence of needle sharing and the viral load of HIV in IDUs. The importance of viral load as a risk factor for parenteral transmission of HIV is demonstrated by the fortunate fact that transmission via accidental needle sticks is not a major cause of infection; most needle sticks do not transfer appreciable amounts of blood. This is in contradistinction to hepatitis B virus infection where 100 million to one billion viral particles per mL of blood are present and the risk of needle stick transmission is substantially increased.
For transmission of HIV through sexual contact, the presence of virus in genital fluids is a key factor. Both seminal and vaginal cells can contain virus as well. It has been suggested that, similar to parenteral transmission, infected cells are the major source of HIV. Consequently, an increased risk of sexual transmission has been seen with advanced disease in infective male partners. Multiple investigations have demonstrated that the presence of other sexually transmitted diseases, ulcerative (syphilis, genital herpes, chancroid) and nonulcerative (gonorrhea, chlamydia, trichomoniasis and bacterial vaginosis) are associated with an increased risk for transmission of HIV).
Prior to the introduction of antiretroviral therapy in the management of HIV-infected pregnant women, maternal-to-infant transmission rates ranged from 13% to 40%. The majority of the cases of maternal-to-child transmission occur during the intrapartum period. In utero or antepartum transmission, although less common, is also well documented. Breastfeeding in the postnatal period has also been shown to be a source for MTCT of HIV. Viral load of HIV has also been demonstrated to be a critically important determinant of MTCT. Thus acute HIV infection with its high level of viral load is a high risk situation for transmission of HIV during the intrapartum period or while breastfeeding. Similarly, advanced HIV disease, which also is associated with high level viremia, carries a high risk for perinatal transmission.
CLINICAL MANIFESTATIONS OF HIV INFECTION
HIV infection produces a wide spectrum of disease. If not treated, HIV infection results in impairment of the immune system, which makes infected individuals susceptible to opportunistic infections (e.g., Pneumocystis jiroveci pneumonia, toxoplasmosis of brain) or neoplasias (e.g., Kaposi’s sarcoma) that rarely occur in the presence of an intact immune system. Although AIDS is the lethal end stage of this spectrum, it is only the tip of the iceberg and represents the minority of HIV-infected individuals. In 1982, the CDC issued a surveillance case definition based on the occurrence of such neoplasias or infections that were moderately to highly predictive of a defect in cell-mediated immunity in the absence of previously recognized predisposing conditions or causes of cell-mediated immune deficiency. Subsequent revisions expanded the list of indicator illness and the case definition included evidence of infection with HIV.
The most recent revision of these criteria occurred in 1993, when the CDC expanded the surveillance case definition of AIDS to include all persons with CD
4 + T lymphocyte count <200 mL, or whose CD
4 + T lymphocyte percentage is <14%
(Table 9.5). Of interest to clinicians providing healthcare for women were the addition of invasive cervical cancer to the list of AIDS defining illnesses (Category C) and several conditions listed in Category B: (i) vulvovaginal candidiasis that is persistent, frequent, or poorly responsive to therapy; (ii) cervical dysplasia (moderate or severe) or carcinoma in situ; and (iii) pelvic inflammatory disease, particularly if complicated by tuboovarian abscesses. Reports indicated that the most common AIDS-defining illnesses in women included:
Pneumocystis carinii pneumonia (PCP); candida esophagitis; cryptococcal meningitis; wasting syndrome, herpes simplex virus bronchitis, pneumonia esophagitis or ulcers >1-month
duration; Cytomegalovirus disease; tuberculosis; CNS toxoplasmosis; and lymphoma.
Pathogenesis of HIV Infection
The pathogenesis of HIV infection has been extensively reviewed by several authors (see
suggested readings).
Figure 9.7 describes the steps involved in the pathogenesis of HIV infection.
There occurs a rapid rise in plasma levels of HIV after infection and widespread dissemination of HIV occurs in association with seeding of lymphoid organs and trapping by circulating dendritic cells. As a result, an impressive viremia occurs with up to one billion virions produced per day. An immune response of similar proportion occurs with high levels of T-cell turnover. Following the initial substantial rise in plasma HIV level (>1 million HIV-1 RNA particles per ml), there is a marked reduction in plasma viremia to a steady-state level of viral replication (set point). This decrease in plasma viremia is the result of virus-specific immune responses that limit viral replication; HIV-1-specific cytotoxic T lymphocytes appear concomitant with the declining titers of HIV.
Within the first month of acute infection, long-lived reservoirs of HIV become established in follicular dendritic cells and resting memory CD4+ T cells. These latently infected cells only produce virus when activated and have a long life span (44 months). These long-lived reservoirs of HIV are a major barrier to HIV eradication with currently available antiretroviral therapies.
During the second to third week of infection, as the viremia rises toward its peak and resting CD4+ T cells are depleted, a hyperactivated state evolves in the immune system. This proliferation and activation of CCR5+ T cells provides the milieu for the extensive viral replication characteristic of HIV infection.
A viral set point, which varies for each individual, is established following the initial drop in HIV viral load. Several factors have been proposed that determine the set point of viral load; these include (i) genetic differences in
coreceptors; (ii) qualitative differences in the immune response; and (iii) differences in the virulence of HIV strains. Lower levels of viremia (set point) are associated with slower disease progression and a better prognosis.
The pathogenesis of HIV infection progresses through three stages: (i) initial acute infection (early stage); (ii) clinically asymptomatic stage; and (iii) symptomatic stage (severe infection, AIDS).
Following the significant decline in viremia at the end of the acute infection stage, HIV-infected persons enter the asymptomatic stage (persistent or latent state), in which virus replication persists, particularly in lymph nodes and peripheral blood mononuclear cells (PBMC), with measured levels in the blood being low. The mechanism by which HIV causes a loss of immune response has been one of the major mysteries of AIDS. Reports demonstrating both high levels of viral production and concomitant rapid production of CD4+ T cells suggest that continuous rapid virus production and CD4+ cell depletion occurs at all stages of infection. As CD4+ cell counts fall below 300 cells/mm3 and high levels of HIV again appear in the blood individuals begin to become symptomatic. At this time a reduction in antiviral CD8 + cell responses also occurs. Ultimately, as the immune system deteriorates, the opportunistic infections and malignancies which define a diagnosis of AIDS appear.
Viral Load and Pathogenesis
The introduction of quantitative methods for measurement of HIV-1 and the subsequent development of quantitative competitive polymerase chain reaction which can measure HIV-1 RNA levels in plasma during clinically asymptomatic and symptomatic stages were major contributors to understanding the pathogenesis of HIV infection. As noted above, this resulted in a new paradigm that detectable, often high levels of HIV-1 replication occurred during asymptomatic as well as symptomatic stages of HIV infection.
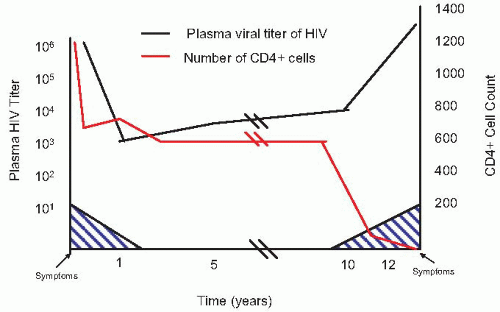
Numerous investigations have documented the critical role that viral load plays in the pathogenesis of HIV infection and in determining clinical outcome as well as response to antiretroviral therapy.
Figure 9.8 demonstrates the relationship between viral load, CD4+ T cell count, and clinical symptoms. The importance of viral load applies to tissue and HIV-infected peripheral blood mononuclear cells (PBMCs) as well as plasma. Plasma HIV-1 RNA level measured after seroconversion is a better predictor of progression to AIDS in men and women than the CD4+ T cell number.
Acute HIV infection
Once HIV antibody testing became available, an acute clinical HIV infection was recognized. Approximately two-thirds of HIV-infected individuals have some symptoms suggestive of an acute retroviral infection. The reported incubation time for acute primary HIV infection ranges from 5 to 35 days, with 10 to 14 days most commonly seen. The illness is characterized by sudden onset, with a duration of 3 to 14 days.
In
Table 9.6 the clinical manifestations of acute HIV infection are listed. The most common signs and symptoms include fever, fatigue, rash (usually maculopapular), headache, lymphadenopathy, pharyngitis, myalgia, arthralgia, aseptic meningitis, weight loss, gastrointestinal distress (nausea, vomiting or diarrhea), night sweats, and oral or genital ulcers.
The symptoms and signs of acute HIV infection are nonspecific and thus diagnosis of the acute illness often poses a challenge for healthcare providers. An important aspect is obtaining an accurate history of exposure. Therefore, a diagnostic workup for acute HIV infection is indicated in patients with signs and symptoms consistent with such a diagnosis and a history of exposure to a person with known or possible HIV infection. Differentiation from infectious mononucleosis (Epstein-Barr
virus) infection is the most common concern. Symptoms very suggestive of acute HIV infection, especially with history of exposure, include a maculopapular rash involving the trunk (40% to 80% of persons with acute HIV infection), acute meningoencephalitis syndrome, and mucocutaneous ulceration involving the oral cavity or genital area.
Typical laboratory findings seen with acute HIV infection include leukopenia with CD4+ lymphopenia and atypical lymphocytosis, mild thrombocytopenia and abnormal liver function tests. A variety of tests are available that detect biomarkers of HIV infection in acute infection. The most common method is documentation of antibody seroconversion. However, this can only be done retrospectively and is the least recommended method. Real-time diagnosis of acute HIV infection is accomplished by the presence of a positive screening test indicating HIV infection (e.g., HIV nucleic acid amplification, HIV p24 antigen, HIV antibody, or combined p24 antigen antibody screening). The availability of rapid HIV-1 antibody tests has been an important advance. Rapid tests are easy to do and provide results as quick as 20 minutes. They can be performed on whole blood, plasma, serum, or saliva. As with other current antibody tests, rapid HIV tests usually become positive within 4 weeks after infection.
Once the diagnosis of acute HIV infection has been confirmed, early treatment with maximally suppressive combination antiretroviral therapy (highly active antiretroviral therapy) should be considered. This recommendation is based on several pieces of evidence including (i) initial viral isolates represent a fairly homogenous swarm of viruses which may be susceptible to combination therapy, (ii) early intervention restores virus-specific cellular immune responses that control viremia; and (iii) early intervention may limit the extent of viral dissemination, restrict damage to the immune system and reduce the possibility of disease progression. Treatment during acute infection may also reduce HIV transmission. In response to this evidence, a panel of experts from the International AIDS Society-USA recommended that immediate therapy be considered for persons with acute HIV infection.
The second (asymptomatic) stage of HIV infection commences several months after the primary acute infection and ranges in duration from as short as 1 year to more than a decade. During the asymptomatic stage, detectable high levels of HIV replication occur; the initial concept that viral infection was latent during this stage is no longer accepted. The length of the asymptomatic stage is probably determined by the host cellular immune response. During the asymptomatic stage, CD4+ T cells begin a slow progressive decline. A concomitant increase in viral load is associated with progression to the final (symptomatic) stage. CD8+ cell function depends on adequate amounts of interleukin-2 (IL-2), which is produced by CD4+ T cells. The decline in CD4+ T cells over time may, therefore, be responsible for the reduction in CD8+ cell antiviral immune responses. As a result of declining CD8+ cell activity, increased replication of a pathogenic HIV isolate leads to the final reduction in CD4+ T cell numbers heralding onset of symptomatic HIV infection.
Symptomatic HIV Infection
Ultimately, in most HIV-infected persons, the reduction in CD4+ T cell level and the concomitant increased viral load result in progression of HIV disease to the symptomatic stage. With CD4+ counts in the 200 to 500 cells/mL range, some individuals will develop mild symptomatic disease. Mild disease is characterized by worsening of chronic skin conditions, recurrent herpes simplex disease, varicella-zoster virus disease, oropharyngeal or vaginal candidiasis, oral hairy leukoplakia, recurrent diarrhea, intermittent fever, or unexplained weight loss. Bacterial infections such as sinusitis, bronchitis or pneumonia are also common. In addition, myalgias, arthralgias, headache, and fatigue are commonly noted.
When CD4+ cell counts fall into the 50 to 200/μL range, patients have advanced immunodeficiency and are classified as having AIDS according to the current (1993) CDC definition. In this advanced stage, OIs such as Pneumocystis jiroveci (previously P. carinii) pneumonia, Toxoplasmosis encephalitis, cryptosporidiosis, isoporosis, tuberculosis, and esophageal candidiasis are frequent occurrences. Among patients who developed symptoms in previous stages, the symptoms worsen. Neurologic complications include neuritis, myelitis, cranial nerve palsies, and peripheral neuropathy. HIV retinopathy with cotton-wool spots may occur. In women invasive cervical carcinoma may occur. Idiopathic thrombocytopenia, anemia and neutropenia also occur.
As CD4+ cell levels fall below 50 cells/μL, patients enter end-stage immunodeficiency. Diseases more likely to occur with end-stage disease include disseminated Mycobacterium avium complex disease, cryptococcal meningitis, progressive multifocal leukoencephalopathy, invasive aspergillosis, disseminated coccidioidomycosis, disseminated histoplasmosis, and invasive Penicillium marneffei disease. Progressive wasting also occurs during end-stage disease.
The 1993, the CDC revised classification system for HIV infection (
Table 9.5) placed symptomatic conditions occurring in an HIV-infected person that are either (i) attributed to HIV infection or are indicative of a defect in cell mediated immune response or (ii) considered to have a clinical course or to require management that is complicated by HIV infection and are not included as an AIDS indicator condition in Clinical category B. Of note, in women are (i) vulvovaginal candidiasis that is persistent, frequent, or poorly responsive to treatment; (ii) cervical dysplasia (moderate to severe) and carcinoma-in situ; and (iii) pelvic inflammatory disease, especially if complicated by tubo-ovarian abscesses. Clinical category C includes the 25 AIDS indicator conditions (
Table 9.5). With the 1993 revision, invasive cervical cancer, pulmonary tuberculosis, and recurrent pneumonia were added to the list of AIDS indicator conditions. HIV-infected persons with significant neurologic disease (without opportunistic infection or malignancy diagnostic of AIDS) are placed in either category B (peripheral neuropathy) or category C (encephalopathy). HIV infection is complicated by a variety of central nervous system disorders that are common and produce significant morbidity. Not only do the neurologic complications result from opportunistic infections and neoplasms, but also from direct nervous system infection by HIV. The neurologic complications of HIV infection generally fall into three categories: (i) AIDS dementia syndrome; (ii) myelopathies; and (iii) peripheral neuropathies.
Patients with AIDS fall into clinical categories C (C1-C3), B3, and A3. The latter two categories are based on CD4+ cell counts <200 m/L or <14% CD4+ cells. Although these classification schemes provide a more precise definition and more accurate extent of HIV infection, the groupings are somewhat artificial in that HIV infection is a continuum that progresses from the acute syndrome through an asymptomatic stage, to ultimate clinical disease and death.
Determinants of HIV Disease Progression
Many clinical and laboratory markers have been used to determine the prognosis of HIV infection. However, recent
investigations have demonstrated that the level of plasma HIV-1 RNA (i.e., viral load) is the single best predictor of the risk of HIV disease progression to AIDS and death caused by AIDS. Although not as reliable a predictor of progression as HIV-1 RNA levels, CD4+ cell counts do provide additional prognostic information. Thus, it is recommended that both plasma HIV-1 RNA levels and CD4+ cell counts should be used to determine the risk of disease progression and response to antiretroviral therapy.
DIAGNOSIS OF HIV INFECTION
As described above, HIV infection encompasses a broad spectrum of disease, ranging from asymptomatic seropositivity to full-blown AIDS. It is characterized by a predictable, progressive derangement of immune function, of which AIDS is a late manifestation. Establishment of a diagnosis of HIV infection prior to the development of symptomatic HIV disease or AIDS is important for several reasons. It allows patients to receive optimal medical care as early in the disease as possible with the resultant opportunity to prevent complications from developing (i.e., prophylaxis against P. carinii pneumonia). Second, there is the potential to use antiretroviral therapy in the early stages of HIV infection to delay progression to AIDS. Third, diagnosis of asymptomatic HIV-infected individuals allows the opportunity to prevent transmission of HIV. Fourth, in pregnant women treatment with antiretrovirals may prevent maternal-to-child transmission of HIV and prevent adverse effects of opportunistic infections on the mother or fetus/newborn.
The majority of HIV-infected adults fall into the asymptomatic infection group of category A. In these individuals, the diagnosis of HIV infection requires laboratory evidence that the virus is present. Diagnostic tests for HIV include antibody testing, antigen detection, viral culture, nucleic acid amplification tests, and immune function tests.
Confirmatory Laboratory
Testing for antibodies against HIV is accomplished by means of enzyme-linked immunoabsorbent assay (ELISA/EIA), immunofluorescent antibody (IFA) or Western blot. The ELISA/EIA for HIV is highly sensitive and specific. Concerns about false-positives with ELISA testing can be alleviated by use of confirmatory tests. Because the positive predictive value of the test depends on the prevalence of HIV in the group being tested (low in the general population) and because of the tremendous medical, social, and psychological impact of a positive HIV antibody test, a confirmatory test for HIV infection is recommended when the ELISA is positive. Additional concern exists over false-negatives, which can be seen in very early HIV infection and late stage AIDS. A “window” (up to 4 weeks after infection) exists early in infection during which time HIV antibody cannot be detected, but virus is present (plasma HIV-1 RNA, proviral DNA, culture or antigen). During this window, the virus can still be transmitted.
The HIV-1 testing algorithm recommended by the Public Health Service comprises initial screening with an FDA-licensed EIA followed by confirmatory testing of repeatedly reactive EIAs with an FDA-approved supplemental test (e.g., Western blot) or IFA. The diagnosis of HIV infection in adults requires that both ELISA/EIA and confirmatory test be positive.
The confirmatory test most commonly used is the Western blot test. This method of gel chromatography isolates the protein in the viral core and envelope (p24 or gp41 bands). Thus, the Western blot test allows determination of the specific antigens against which antibodies are directed. In general, positive bands from two or the three major antigen groups, the gag, pol or env region of the virus are required for a positive test. The CDC criteria require the presence of at least two of the following bands: p24, gp 41, or gp 160/120 for a positive result. False-positive Western blot results are extremely uncommon. Indeterminate Western blot results do occur and are caused by incomplete antibody response to HIV or nonspecific reactions in sera of uninfected persons. Incomplete antibody responses may occur in persons recently infected with HIV who are seroconverting, persons with end-stage disease and perinatally exposed infants who are seroconverting (i.e., losing maternal antibody). Nonspecific reactions causing intermediate results occur more frequently among pregnant or parous women than among other individuals in low HIV seroprevalence groups. The IFA tests, which use HIV-infected T cells as antigen, are not widely used because of the time, expense, and expertise required. However, IFA can be used to resolve an EIA-positive, Western blot-indeterminate specimen.
Viral culture for HIV is expensive, slow (>1 month), and its sensitivity is unknown. Because of these characteristics, viral culture for HIV has not been used extensively in routine clinical care of HIV-infected persons. Moreover, special biocontaminant facilities are required for working with HIV.
Several methods and test kits have been developed for the detection of HIV antigen. In general, these tests detect the p24 and gp 41 antigens of HIV. HIV antigenemia mirrors HIV-1 RNA levels and is biphasic—an initial high level prior to seroconversion and a reappearance of antigen late in the disease process as the immune system is depleted. The introduction of monoclonal anti-p24 antibodies significantly increased the sensitivity of the p24 antigen assay with levels as low as 7 to 10 pg/mL being detected; an additional modification the acidified p 24 antigen procedure, further enhanced the sensitivity of p 24 antigen testing. Acidification of plasma disrupts the antigen-antibody complex and releases free p24 antigen for detection.
The introduction of polymerase chain reaction (PCR) for the detection of HIV was an important addition to our battery of HIV detection tests. PCR is an amplification technique for viral DNA and allows rapid detection of small amounts of HIV. This technique can detect as few as one copy of HIV per 100,000 cells. Application of the PCR technique for early diagnosis of HIV infection of the newborn was a significant advance. In the case of HIV, proviral HIV-1 DNA, genomic RNA, and mRNA have all been amplified successfully. The major drawback to PCR is its phenomenal sensitivity and thus very small amounts of contamination can result in false-positive results. Techniques have been developed to quantitate the amount of HIV proviral DNA and genomic RNA as measures of viral load. Competitive PCR techniques have also shown great promise. Extracellular HIV-1 RNA rises to high levels shortly after infection in adults and in infants. Thus, the sensitivity of tests detecting HIV-1 RNA is excellent for diagnosis of acute and chronic HIV infection in adults and early diagnosis of perinatally acquired HIV infection.
Rapid HIV testing is also now available; results can be available in <20 minutes. The sensitivity and specificity of these assays are comparable to those of ELISA/EIA (i.e., sensitivity, 100%; specificity, 99%). However, the positive predictive value varies according to the prevalence of HIV infection in the population tested. Thus, the positive predictive value is low in low prevalence populations resulting in many false positive results. As a consequence reactive rapid HIV tests must also be confirmed. Probably, the greatest utility of rapid HIV testing is among pregnant women in labor whose HIV status is unknown, especially in areas where the prevalence of HIV is high
or the mother is high risk for HIV infection. Such an approach would identify those pregnant women whose infants might benefit from intrapartum and postdelivery administration of antiretroviral therapy. Similarly, rapid tests can be utilized on newborns to determine their HIV exposure. Consideration should be given to having rapid HIV testing available to patients in hospitals, where the prevalence of HIV is high and the incidence of no prenatal care is high.
EPIDEMIOLOGY OF HIV INFECTION IN WOMEN
In the United States, as of December 2006, there had been 189,566 women with AIDS reported to the CDC. Women account for over 18% of all cumulative reported AIDS cases, a substantial increase from 8% of cases reported from 1981 to 1987. Women have the fastest rate of increase in HIV infection in the United States and over 25% of new HIV and AIDS cases are women. According to the CDC approximately one-third of people living with HIV in the United States are women.
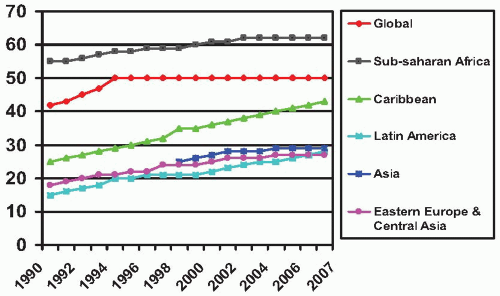
Figure 9.9 demonstrates the growing role of women in the AIDS epidemic in the United States. AIDS is currently the third leading cause of death in all women in the United States and the leading cause of death among Africa-American women. In the United States, AIDS, especially heterosexually acquired, occurs disproportionately among women of color, with over 80% in African-American or Hispanic women
(Table 9.7).On a global basis women account for 50% of the more than 60 million adults estimated to have been infected with HIV
(Fig. 9.10). In sub-Saharan Africa, over 60% of adults living with HIV in 2007 were women. The proportion of women living with HIV in Latin America, Asia, and Eastern Europe are slowly growing. In Eastern Europe and Central Asia it is estimated that women accounted for 26% of adults with HIV in 2007 (compared with 21% in 2001). In Asia, 29% of HIV infected persons are women (up from 26% in 2001). Even though the proportion of women has been increasing in each region, in most regions (except sub-Saharan Africa) the overall number of men infected still far outnumbers that of women.
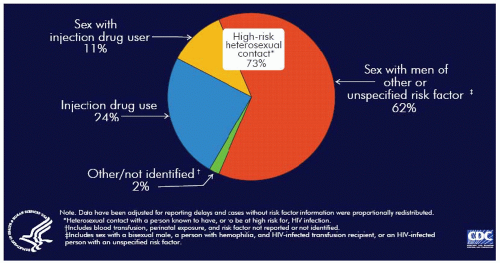
Worldwide heterosexual transmission is the predominant mode of HIV transmission. Although in the United States MSM remains the largest category of transmission, among women, heterosexual transmission is the largest group (62%), followed by IDU (24%). Including heterosexual contact with IDUs, 73% of AIDS cases currently reported in women in the United States arise from high-risk heterosexual contact
(Fig. 9.11). In the 33 states with name-base HIV infection reporting, high-risk heterosexual contact accounts for 80% of HIV/AIDS cases (
Fig. 9.11).
Transmissibility
The risk for acquiring HIV infection from a single or even multiple sexual acts with an infected individual is not known. There is general agreement that HIV is less easily transmitted than other STD organisms such as Neisseria gonorrhoeae, Chlamydia trachomatis, and Treponema palladium. Worldwide, unprotected heterosexual intercourse is the major risk factor for HIV infection in women and adolescents. Among discordant heterosexual partners, male-to-female transmission of HIV is relatively more efficient than female-to-male transmission with estimates of this increased risk ranging from 2- to 17-fold.
Risk factors that are associated with heterosexual transmission have been suggested. These include lack of condom use, presence of other STDs (ulcerative and nonulcerative), acute HIV infection, advanced disease state (according to CD4+ cell count, viral load or AIDS diagnosis), and cervical ectopy.
HIV and Contraception
Initially, there were concerns regarding the role of intrauterine contraceptive (IUD) use and hormonal contraception in increasing the risk of HIV transmission. Most HIV-infected women are of reproductive age and preventing unwanted pregnancies plays an important role in prevention of maternal-to-child transmission of HIV. Although condoms can effectively prevent HIV transmission, they are not highly effective at preventing pregnancies (because of improper use).
In theory hormonal contraceptive use could increase the risk for HIV infection secondary to increased cervical ectopy, local effects of progesterone which thin the vaginal epithelium, and changes in cervical mucus. In non-human primates progesterone implants led to vaginal wall thinning and a nearly eightfold increased risk of acquiring SIV, most likely because of thinning of the vaginal epithelium resulting in loss of the physical barrier of the vaginal wall, exposing and rendering dendritic (Langerhan’s) cells in the vaginal epithelium more susceptible to HIV infection. In addition, progesterone may act to down regulate cell mediated immune effectors (i.e., cytotoxic CD8+ lymphocytes). In another study of humans both medroxy progesterone acetate (Depo-Provera) and oral contraceptives were associated with increased viral shedding from the cervix (even after controlling for low CD4+ cell counts).
Although oral contraception effectively prevents pregnancies, it does not protect against HIV and other STDs. In addition, significant drug interactions with antiretroviral therapy have been demonstrated with oral contraceptives. The antiretroviral agents (ARTs) nevirapine, lopinavir/ritonavir, nelfinavir, and ritonavir decrease levels of ethinyl estradiol (20% to 47%); nelfinavir also decrease norethindrone levels 18%. These decreases may affect the efficacy of oral contraceptives. On the other hand, atazanavir, indinavir, and efavirenz increase levels of ethinyl estradiol and norethindrone, which could increase the risk of side effects. Hormonal contraception decreases the levels of the ART amprenavir and thus should not be given together. Medroxyprogesterone (Depo-Provera) may be an option as it is not affected by ARTs.
IUDs provide a high degree of efficacy for preventing pregnancies. Initial observations suggested increased complications and decreased efficacy among HIV-infected women. However, more recent reports have shown that the currently available IUDs are equally safe in HIV-infected and HIV-uninfected women and that use of IUDs was not associated with increased levels of HIV-1 DNA genital shedding.
HIV and Viral Load
The role of viral load in the risk of sexual transmission has been the focus of intense investigative effort. Generally, the viral load in semen correlates with the viral load in plasma. Transmission among heterosexuals is associated with rapidly replicating virus (in tissue culture) and increased plasma viral load (>15,000 HIV-1 RNA copies per milliliter). Administration of potent antiretroviral therapy substantially reduces HIV viral load in semen and vaginal secretions. Although such a decrease could potentially reduce the risk of sexual transmission, antiretroviral therapy should not be considered an effective means of preventing sexual transmission of HIV. Until recently it has been unclear whether differences existed between men and women for the viral load of HIV-1 RNA in plasma. Several studies demonstrated lower levels in women than men, after controlling for CD4+ lymphocyte counts. On the other hand, no difference was noted in other studies.
The viral load of HIV-1 RNA following seroconversion (set point) is a strong independent predictor of the risk of progression to AIDS. As a result of these investigations, viral load has formed the basis on which the current guidelines for initiation of antiretroviral therapy, which apply similarly to women and men, have been formulated.
HIV and Sexually Transmitted Disease
Sexually transmitted diseases, especially those that manifest as genital ulcers, have been strongly associated with an increased risk for HIV infection. Initially, an association was demonstrated between sexually transmitted genital ulcer disease (e.g., syphilis, genital HSV-2, and chancroid) and HIV infection. Genital ulceration facilitates acquisition and/or transmission of HIV in two ways. Firstly, ulceration provides a more accessible portal of entry or exit for HIV. Second, the immune and inflammatory response to genital ulcers (especially HSV and syphilis) results in activated macrophages and stimulated T-lymphocytes. Not only are such stimulated cells more susceptible to HIV, but such stimulation of latent HIV-infected T lymphocytes results in activation of and increased shedding of HIV. In addition, nonulcerative STDs (gonorrhea, chlamydia, trichomoniasis, and bacterial vaginosis) have been associated with an increased risk for HIV infection. Nonulcerative STDs are associated with an inflammatory response (i.e., proinflammatory cytokines) which upregulates production of HIV-1. This results in higher viral titers. Studies have demonstrated that genital tract infections increase the shedding of HIV-1. The semen of men with urethritis (gonococcal more so than chlamydial) contained higher levels of HIV-1 RNA than that of HIV-infected men without urethritis. Similarly, increased levels of cervicovaginal shedding of HIV among women coinfected with STDs has been shown. Treatment of STD (nonulcerative and ulcerative) reduced shedding of HIV, although shedding was not eliminated. As a consequence of this increased HIV shedding, the likelihood increases that genital secretions will contain viral load of HIV required for transmission. Contrary to the situation where HIV infected women are not co-infected with other STDs and where the rate of female-to-male transmission is less efficient, if women have another STD, female-to-male transmission is as likely as male-to-female transmission. Because of their prevalence in sexually active women, genital herpes (HSV-2) and bacterial vaginosis (BV) play an important role in the transmission of HIV. It has also been suggested that the depletion of immune function associated with HIV infection could worsen the
clinical course of some STDs (e.g., genital herpes and syphilis) and/or affect the efficacy of treatment.
An association of BV and acquisition of HIV has been reported in multiple studies. In cross-sectional studies, women with BV had a higher prevalence of HIV infection, with an odds ratio of 1.5 to 2.5. Longitudinal studies have also demonstrated that women with BV have an increased risk to acquire HIV. In a study from Malawi, pregnant women with clinical BV had an increased risk for HIV-1 seroconversion during pregnancy and in the postpartum period; antenatal seroconversion odds ratio was 3.7 (p = .04) and postpartum adjusted rate ratio was 2.3. In a prospective cohort study examining the relationship between vaginal colonization with lactobacilli, BV, and acquisition of HIV, absence of vaginal lactobacilli was associated with an increased risk of acquiring HIV-1 infection (hazard ratio [HR] 2.0; 95% CI, 1.2 to 3.5) and presence of abnormal flora on Gram’s stain was also associated with increased risk of HIV-1 acquisition (HR 1.9; 95% CI, 1.1 to 3.1). A study of HIV-infected women demonstrated that women with BV were significantly more likely to have HIV RNA detected in the genital tract (78%) than those HIV-infected women without BV (34%). Potential mechanisms to explain the association of BV and HIV include (i) peroxide producing lactobacilli predominate in the normal vaginal flora and maintain a low pH; (ii) peroxide producing lactobacilli demonstrate viricidal effects on HIV-1; (iii) combining peroxidases and halide ion (both present in the vagina) with peroxide enhances the viricidal activity from 50% (peroxide alone) to nearly 100%; and (iv) low vaginal pH (as seen with normal flora) inhibits CD4+ lymphocyte activation and may reduce the number of target cells for HIV-1 in the vagina.
An increasing literature has emerged assessing the association between HSV and HIV infections. HSV infections are common in HIV-infected women (50% to 90%). As in non HIV-infected women approximately 70% of women with HSV infection are asymptomatic. However, even these asymptomatically infected patients frequently shed HSV in their genital tract. Individuals coinfected with HSV-2 and HIV have more frequent episodes and higher viral levels of HSV-2 shedding from the genital tract mucosa. Moreover, genital tract shedding of HSV correlates with HIV viral load and is inversely related to CD4 cell count.
The clinical presentation of genital herpes may be affected by coinfection with HIV. Symptomatic genital herpes in women coinfected with HIV varies widely from the typical small, confined ulcerative lesions seen in the general population to extensive, deeply ulcerated and necrotic lesions. With HIV coinfection, the lesions of HSV-2 may occur in unusual locations.
A recent study from the University of Washington demonstrated that asymptomatic as well as symptomatic HSV-2 reactivation results in increased plasma levels of HIV. In women not taking HAART, valacyclovir suppression of HSV resulted in significant reduction in genital tract shedding of HIV. In addition, a meta-analysis of eight clinical trials, including nearly 1,800 patients, noted that acyclovir suppressive therapy led to a significant survival benefit in women (and men). Again this effect is primarily limited to individuals not on HAART.
Two important, community-based intervention trials were performed to assess what impact STD prevention and control would exert on HIV infection rates. The initial study in Mwanza, Tanzania focused on enhanced syndromic diagnosis and treatment of symptomatic STDs in an area where the HIV epidemic was in its early stages with an estimated HIV seroprevalence of 4%. In this study HIV incidence was reduced by 42% after 2 years. The second study focused on community based treatment of all members, including those with symptomatic and asymptomatic STDs in the Raki district of Uganda, an area where the AIDS epidemic was well established, with 16% of the study population HIV-seropositive. The Raki study found that HIV infections were not prevented by the intervention. Thus, although STD interventions may be effective early in the AIDS epidemic, once the epidemic has matured beyond phase 1 (rapid infection of very susceptible infection), short-term STD prevention and control will probably have very little impact on the AIDS epidemic.
Transmission via Blood/Blood Products
Transmission of HIV via blood occurs by several mechanisms. Currently, <1% of adults with AIDS in the United States acquired HIV infection from transfusion of blood or blood products. Whereas whole blood, blood cellular components, plasma, and clotting factors have been shown to transmit HIV infection, no products prepared from immunoglobulin, albumin, or plasma protein fraction have been demonstrated to do so. Individuals at risk for transfusion-associated HIV infection are those who received blood between 1978 and April 1985 when screening donated blood and plasma for HIV antibody was instituted. Such screening and heat treatment of clottingfactor concentrates have significantly reduced the risk of acquiring HIV via transfused blood products. It is estimated that the risk for HIV infection being acquired from a transfused unit of blood is between 1 in 100,000 and 1 in 1,000,000.
Second, parenteral inoculation of blood may transmit HIV. Fortunately, the parenteral route is associated with a very low risk of transmission. This low risk is probably caused by the very small inoculum of blood associated with single needle stick injuries. The risk of occupational HIV transmission has been assessed in prospective studies of exposed healthcare workers. Pooled data demonstrate that the average risk of HIV transmission associated with needle punctures or similar percutaneous injuries is 0.32%. For mucocutaneous transmission the estimated risk is 0.09%. Studies have shown that no infection occurred from (i) routine (nonparenteral) contact with HIV-infected patients; (ii) aerosolization or airborne droplets; (iii) cutaneous exposure involving intact skin; or (iv) contact with contaminated environmental surfaces or fomites. Thus, it appears that only needle sticks with blood from HIV-infected individuals have been associated with infection in the study of healthcare workers.
The inoculum of virus transmitted during exposure appears to be the critical HIV seroconversion in healthcare workers after percutaneous exposures. Deep intramuscular penetrations, large-bore hollow needles, and injections of blood are the factors associated with most of the needle stick injuries that result in occupational acquired HIV infections. Deep punctures (i.e., skin penetrated resulting in spontaneous bleeding), injuries caused by devices visibly contaminated with blood, and injuries associated with devices that had entered a blood vessel were found to be independent factors associated with risk for acquisition of HIV.
Transmission in Injection Drug Users
The third method (and by far the most difficult to manage) for transmission by blood is that caused by injection drug use. As of December 2006, 25% of AIDS cases in the United States have occurred in IDUs. Among women with AIDS, 19% are IDUs. The importance of IDU is dramatized by the recognition that IDUs are the largest pool of HIV-infected heterosexuals in the United States and that IDUs and partners of male IDUs are the major source of perinatal AIDS. The major risk
factor for transmission of HIV in association with IDU is the sharing of unsterilized needles and syringes contaminated with blood infected with HIV.
Maternal-to-Child Transmission
Three routes for maternal-child transmission of HIV have been proposed. These are (i) in utero transplacental transmission from maternal circulation; (ii) intrapartum transmission during the process of labor and delivery by inoculation or ingestion of blood or other infected fluids such as amniotic fluid; or genital secretions; and (iii) postpartum transmission via ingestion of infected breast milk.
A bimodal model of in utero and intrapartum transmission appears to exist. Infants infected in utero have virus detected by culture or PCR within 48 h of birth. The presence of in utero transmission is supported by studies demonstrating HIV-1 in fetal tissues as early as 8 weeks gestation and in fetal thymus tissue as early as 15 weeks. According to the Institute of Medicine, at most 25% to 30% of perinatal HIV transmission occurs in utero. Indirect evidence suggests that 70% to 75% or vertical transmission of HIV occurs intrapartum.
The rate of perinatal transmission of HIV varies by geographic region. This risk will be discussed in detail in the next section. Briefly summarized, in the United States and Western Europe prior to widespread use of antiretroviral therapy transmission rates ranged from 14% to 33%. In the developing world, rates as high as 43% have been reported.
Clinical Manifestations of HIV Infection in Women
As previously noted, HIV infection encompasses a spectrum ranging from asymptomatic infection to a severe immunodeficiency state associated with opportunistic infections, neoplasms, and other manifestations (e.g., HIV encephalopathy, HIV wasting syndrome). Primary HIV infection may present as an acute mononucleosis-like illness as described previously. An estimated 40% to 90% of patients with primary infection have symptoms. The interval between exposure and symptomatic illness, on average, ranges from 2 to 4 weeks and the duration of clinical illness is 1 to 2 weeks. Following resolution of primary infection, the patient enters the asymptomatic stage.
In the absence of antiretroviral therapy, HIV infection progresses, over time, to a state of severe immunosuppression characterized by high viral titers and low CD4+ T lymphocyte levels and development of AIDS. Certain clinical and laboratory indicators appear to be predictive of progression of HIV infection. Clinical findings associated with an increased risk for development of AIDS include oral candidiasis, oral hairy leukoplakia, and severe recurrent herpes zoster. Decreased levels of CD4+ T lymphocytes (<500 cells/mL) identify patients with HIV-related immunosuppression and are excellent predictors of progression of HIV infection. The viral load (quantity of HIV-RNA is serum or plasma) is the single best predictor of progression to AIDS and mortality. Utilizing both of these laboratory tests enhances the prognostic accuracy for progressive disease.
Table 9.5 is a list of AIDS-indicator diseases. Since 1996 there has been a continual decline in AIDS-defining opportunistic illness in the United States. In large part this is caused by the availability of antiretroviral therapy, especially HAART, and prophylaxis against OIs when indicated by level of immunosuppression.
Factors that may influence the clinical presentation of HIV infection or the response to therapy in women have been assessed. They include altered pharmacokinetics of drugs secondary to gender or interactions between commonly used drugs and antiretrovirals and differences in the immune system. In general, the clinical manifestations of HIV disease are similar for both men and women. However, there are gender differences related to the occurrence of specific AIDS-defining OIs or malignancies. For example, HIV-infected women have lower rates of Kaposi’s sarcoma than men and they have higher rates of recurrent bacterial pneumonia with encapsulated bacteria (e.g., Streptococcus pneumoniae, Haemophilus influenzae). In addition, increased rates of specific diseases of the female genital tract which are related to HIV infection are seen in HIV-infected women. These include higher rates of cervical dysplasia with accelerated progression to invasive cervical cancer and recurrent or persistent vulvovaginal candidiasis.
The initial clinical manifestations of HIV infection in women has been the focus of multiple studies. Studies prior to the availability of HAART did not identify any gender differences in the frequency of AIDS-defining OIs. Overall, three clinical manifestations accounted for over 75% of reported AIDS-indicator conditions in the pre-HAART era: P. jiroveci (previously P. carinii) pneumonia (42%), HIV wasting syndrome (20%), and esophageal candidiasis (15%). In several studies, esophageal candidiasis was the most common AIDS-defining manifestation in women followed by PCP. However, other investigations have failed to confirm this finding and have not noted any gender differences in the frequency of esophageal candidiasis or other AIDS-defining OIs. Other OIs commonly seen in HIV-infected women include CMV, recurrent mucocutaneous HSV, mucocutaneous candidiasis, toxoplasmosis and tuberculosis.
Human papillomaviruses (HPV), CIN, and associated cancers appear to behave differently in HIV-infected women, especially as cell-mediated immunity is impaired. HPV and cervical neoplasia are discussed in the section “Gynecologic Aspects of HIV Infection.”