With the development first of nucleoside reverse transcriptase inhibitors starting in the mid-1980s2 and subsequently of protease inhibitors and nonnucleoside reverse transcriptase inhibitors, combination antiretroviral therapy (cART) involving three or more drugs became broadly available in 1996.3 There are now 30+ antiretroviral agents approved that target one of several stages of the HIV lifecycle.4 HIV suppression with cART leads to increases in CD4 lymphocyte counts, improved immune function, decreased immune activation, decreased infectious complications, decreased mortality, and decreased infectivity,5–10 transforming HIV infection into a manageable chronic disease.
The availability of cART has also transformed cancer epidemiology in people with HIV. Although AIDS-defining tumors remain the most common cancers, the incidence of KS and NHL decreased after cART became widely available in the United States and other developed countries.11 Additionally, over the past 15+ years, therapy for major AIDS-associated cancers has also improved.12 At the same time, the number of persons with HIV in the United States has increased by >50% due to an estimated 40,000 to 50,000 new HIV infections each year11,13 and a decrease in infectious deaths. Worldwide, >30 million people are infected with HIV. With HIV-infected individuals living longer, the population at risk for malignant complications has increased and is aging.14 In addition to AIDS-defining malignancies, patients infected with HIV are at increased risk of certain other cancers, including classical Hodgkin lymphoma (cHL), lung, anal, head and neck, liver, and nonmelanoma skin cancer.15 Other common cancers such as breast or colon cancer are not more frequent in patients infected with HIV; even so, the burden of these cancers is increasing as the HIV-infected population ages. As such, the cumulative risk of developing non-AIDS defining malignancies (NADM) (Table 117.2) is an increasing public health concern.7,16–19 Until there are improved HIV prevention strategies10,20 or a broadly available HIV cure,21 cancer burden will likely continue to increase substantially. Given these epidemiologic trends, oncologists will be called on to manage more cancer in people with HIV.
CANCER AND HIV: INCIDENCE AND ETIOLOGY
Epidemiologic studies have led to important discoveries regarding pathogenesis of HIV-associated tumors. KS in AIDS had a different natural history than the form in elderly men described by Moritz Kaposi in 187222 and occurred in a specific subpopulation of patients with AIDS: young men who had sex with other men (MSM).23,24 MSM without HIV also occasionally developed KS. This suggested that KS had an infectious cause other than HIV itself, and ultimately a novel gamma-herpesvirus, KS-associated herpes virus (KSHV) or human herpes virus (HHV)-8, was discovered in 1994.25 KSHV is an essential causative agent for all epidemiologic forms of KS. Seroprevalence of KSHV parallels geographic incidence of KS.26 In the United States, KSHV seroprevalence is high in MSM but is <10% in the general population.27
KS epidemiology has evolved with the changing AIDS epidemic. In the United States prior to 1985, KS was the initial manifestation of AIDS in approximately 30% of cases.28 However, after peaking in the early 1990s, its incidence declined in developed countries with the introduction of nucleoside antiretrovirals and then cART. In the United States, the incidence of AIDS-associated KS decreased by 84% in the period 1996 to 2002 compared with 1990 to 1995.29 Further declines since 2002 have been more modest, with incidence relatively stable at about 62 cases per 100,000 person years in recent years.11 It remains the second most common tumor in people with HIV/AIDS in the United States,11,18 In regions such as sub-Saharan Africa, where KSHV and HIV infection are each highly prevalent, KS is a substantial public health burden.30,31 In parts of sub-Saharan Africa, KS has been reported to be the most common tumor in men, representing almost half of all cancers, and also the second most frequent tumor in women.31–33 Increasing access to cART via programs such as the Joint United Nations Programme on HIV/AIDS and the US President’s Emergency Plan for AIDS Relief appears to be decreasing KS incidence in parts of sub-Saharan Africa,34 but the extent of this change remains to be defined.
Excess cases of NHL were also noted early in the AIDS epidemic, and certain NHLs were included in the 1985 AIDS case definition.35 In the CDC 1993 definition of AIDS, three NHLs were considered AIDS-defining: Burkitt (or equivalent), immunoblastic (or equivalent), or primary brain.1 This terminology is now dated; however, several lymphomas remain strongly associated with HIV. In this chapter, we use the term AIDS-related lymphoma (ARL) when referring to epidemiologic data that does not segregate the various lymphoid tumors (see Table 117.1).36 However, ARL is no longer a clinically useful term, and lymphomas should be classified using current World Health Organization criteria.36 For AIDS-defining lymphomas, risk in patients infected with HIV in the pre-cART era was more than 250-fold greater than the general population. Most lymphomas that occur in patients infected with HIV are high-grade mature B-cell lymphomas,37 including diffuse large B-cell lymphoma (DLBCL) with either centroblastic (germinal center [GC]) or immunoblastic (activated B-cell [ABC]) phenotypes,38 Burkitt lymphoma (BL), plasmablastic lymphoma (PBL), primary diffuse large B-cell lymphoma of the central nervous system (PCNSL), and primary effusion lymphoma (PEL). By contrast, these histologies make up 25% of NHL in the general population.39 ARL risk increases with the degree and duration of immunosuppression, and is inversely related to CD4 count.40 Inflammation41–44 and uncontrolled HIV viremia45–48 also contribute to lymphoma risk. Since the advent of cART, NHL incidence has decreased by 50%.49 Overall, populations on cART have improved immune status and CD4 counts at levels where lymphoma standardized risk ratio is lower. Still, within any CD4 cell strata, the standardized risk ratio for lymphoma remains elevated in individuals infected with HIV and comparable between pre-cART and early cART eras.49 Indeed, changes in NHL incidence partially reflect changes in the proportion living with a certain CD4+ cell level. Despite a decrease in incidence, with an estimated incidence of 97 per 100,000 person-years in the cART era, NHL is the commonest malignancy in individuals infected with HIV in the United States.18 ARL is an increasing public health problem in areas of sub-Saharan African with high HIV prevalence.50,51
With cART, epidemiologic and clinical patterns of ARL have changed. In the United States, ARL survival has increased since introduction of cART by nearly two-fold,12,29,52 due in part to the effect of cART on AIDS and its infectious complications, as well as a shift away from poor-prognosis histologies that tend occur with advanced AIDS.53 This is most remarkable for PCNSL, which generally occurs in patients with <50 CD4 cells/mm3, and whose incidence has fallen 80%.29 For systemic ARL, immunoblastic subtypes predominated in the pre-cART era, but the predominant subtypes emerging in areas where cART is widely available are those with the GC phenotype of DLBCL and BL,49,54–56 which are associated with longer survival.57 Lymphoma therapeutic advances also contribute to improved survival. With current regimens, prognosis for the most common HIV-associated lymphomas approach that of the general population. This is particularly apparent for the GC phenotype of DLBCL55 and BL.52 Outcomes in PCNSL remain poor,53,58 in part due to difficulty in diagnosis, neurologic comorbidities, lack of standardized therapy, and delayed or inadequate treatment of lymphoma.59
While the incidence of ARL and KS has decreased, the incidence of certain NADMs has increased. Where cART is widely available, NADMs now occur as frequently as AIDS-defining malignancies.19,60 Furthermore, cancer has emerged as the leading cause of death in people with HIV,7,58,60–62 with about half of cancer-associated deaths caused by AIDS-defining malignancies and half by NADM.7,63 Excess risk of certain NADMs is partly attributable to patients infected with HIV having increased infection with, and in some cases, poor immunologic control of oncogenic pathogens, such as human papillomavirus (HPV), Epstein-Barr virus (EBV), hepatitis B virus (HBV), and hepatitis C virus. It is possible that additional infectious agents will be discovered and found responsible for some of these tumors. For example, a novel virus, Merkel cell polyomavirus, was recently identified as the etiologic agent for Merkel cell carcinoma,64 a rare skin cancer that occurs with increased frequency in immunosuppressed individuals, including those with HIV.65 Other risk factors for certain of these tumors are more common in patients with HIV, including cigarette smoking and alcohol use. For many of these tumors, there is evidence that HIV also plays a pathogenic role, although the mechanisms may be complex or indirect. For example, a nearly five-fold excessive lung cancer risk in HIV appears related to age and cART use, but not CD4 cell count, and the risk remains approximately two-fold greater than that of the general population even when adjusted for smoking.66,67 A contributing factor increasing the risk of lung cancer in people with HIV may be recurrent lung infections.67,68
Pathophysiology
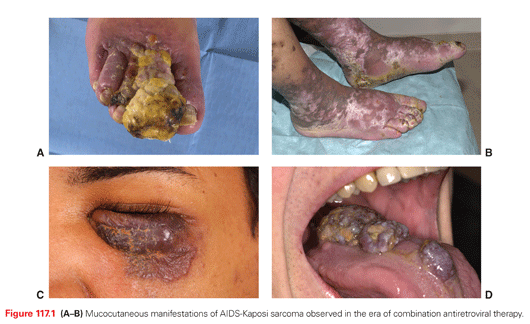
KS is a multifocal angioproliferative tumor caused by KSHV. Its characteristic purplish lesions are found histologically to have proliferating spindle cells with markers of lymphatic endothelial cells, leaky vascular slits, and an inflammatory infiltrate.69–71 KS spindle cells are generally considered polyclonal or oligoclonal, although advanced lesions may have elements of monoclonality.72,73 The lesions are most commonly cutaneous, but may involve internal organs including the lungs, lymphatic system, and gastrointestinal tract (Fig. 117.1).
KSHV is a necessary cause of KS. In the absence of cofactors such as HIV or iatrogenic immunosuppression, the risk of KS among individuals infected with KSHV is relatively low. KS was initially categorized based on epidemiologic characteristics. As first described by Moritz Kaposi in 1872,22 KS was an indolent tumor usually seen in elderly Mediterranean men (now called classical KS). KS also exists in parts of Africa, where KSHV seroprevalence is 40% to 80% (endemic KS, seen in men, women, and occasionally young patients), in immunosuppressed populations (i.e., transplant recipients [iatrogenic KS]), and persons with HIV (epidemic KS). Clinical behavior varies between epidemiologic subtypes and may be related to etiology of underlying immunodeficiency and presence of other KSHV-associated diseases.
Hyperproliferation of KSHV-infected spindle cells is the hallmark of KS.74–76 KSHV modifies host cellular pathways by multiple mechanisms, in part through expression of KSHV-encoded mimics of human genes, viral microRNA, and activation of cellular genes.77 Through these mechanisms, KSHV activates prosurvival and proangiogenic pathways. In particular, a KSHV-encoded G-protein–coupled receptor (vGPCR, also known as ORF74) induces production of vascular endothelial growth factor (VEGF) and other angiogenic factors.75,78–80 Other important viral proteins include homologues of a macrophage inhibitory protein with angiogenic activity74,81 and a viral interleukin (IL)-6 (vIL-6) with inflammatory and prosurvival effects. KSHV also induces c-Kit and endothelial-to-mesenchymal transformation.82,83 These and overproduction of human cytokines and growth factors such as VEGF and platelet derived growth factor by KSHV-infected cells contribute to spindle cell proliferation and angiogenesis.
The marked elevation in KS in immunosuppressed individuals suggests an important role for defective immune surveillance of KSHV in risk of KS pathogenesis.84,85 Decreasing CD4 cell count is associated with increasing risk of KS in both AIDS-associated and classic KS.86,87 In patients with controlled HIV, KS is associated with a T-cell immunosenescense phenotype marked by increased CD57+ and decreased CD28+ T cells.88 Patients with KS have decreased KSHV-specific CD4 responses compared to HIV/KSHV co-infected individuals without KS.85 KS response to immune modulation is associated with increases in absolute CD4 count and/or KSHV-specific T-cell responses in patients infected with HIV on cART and organ transplant recipients after reduction in immunosuppression.89–91 HIV may promote KS by other mechanisms as well. For example, the Tat protein of HIV can enhance infection of target cells by KSHV.92
Staging and Prognosis
KS is a multifocal tumor without evidence of clonal expansion and dissemination. KS can arise simultaneously at multiple sites (see Fig. 117.1) but should not be described as metastatic. Standard oncologic staging and response criteria are not applicable. The most widely used staging system for KS is the AIDS Clinical Trials Group Oncology Committee TIS staging system93,94 (Table 117.3). Risk stratification is based on tumor burden (T0 or 1), immune status (I0 or 1), and presence of any systemic illness (S0 or 1). For each category, poor risk (subscript 1) is defined respectively by the presence or extensive cutaneous or oral disease, tumor-associated edema, ulceration, or visceral disease (T1); CD4 <150 cells/mm3 (I1); and the presence of other opportunistic infections, constitutional symptoms, or poor performance status (S1). Before cART, poor risk in one or more categories was considered poor risk. In the cART era, a baseline CD4 cutoff of 150 cells/mm3 is not as important prognostically,95 and two main risk categories have been defined: good risk (T0S0, T1S0, or T0S1) and poor risk (T1S1). Notably, pulmonary involvement carries a particularly elevated risk of death.96 Also, women who develop HIV-associated KS have a worse prognosis independent of other TIS risk factors.95,97,98
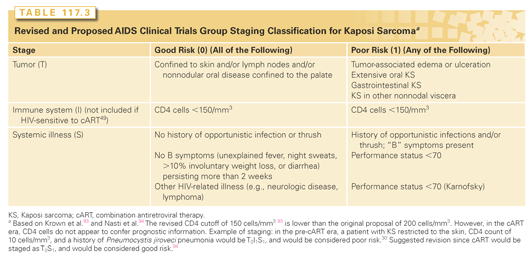
In assessing KS response to therapy, most clinical trials use some modification of criteria established by the AIDS Clinical Trials Group Oncology Committee.99 Criteria for a partial response include 50% decrease in the total number of prespecified indicator lesions, 50% decrease in the area of measured cutaneous lesions, or flattening of 50% of nodular indicator lesions in the absence of progressive disease. Complete response requires resolution of all measurable disease, although residual skin hyperpigmentation is common. Outside of the research setting, KS is commonly treated to a symptomatic endpoint or response plateau.
Treatment
Treatment with cART is fundamental to therapy of HIV-associated KS, and generally should be the first therapeutic maneuver. Drugs associated with iatrogenic KS, such as steroids, cyclosporine, and rituximab, should be avoided or minimized.100,101 KS-specific therapy is reserved for select cases, in particular patients with advanced (T1) KS who generally will not have adequate and timely responses to cART alone or patients who have some other need for a rapid response. In patients with pulmonary KS, chemotherapy may be required urgently. Also, specific KS therapy is used in patients with KS unrelated to HIV that requires therapy, or transplant patients that do not respond to changes in the immunosuppressive regimen.
Regression of KS with cART alone has been documented in up to 80% of cART-naïve patients with early disease (T0).95,102,103 Control of HIV viremia and immune reconstitution are key determinants of the anti-KS effect of cART. Some laboratory studies have suggested that HIV protease inhibitors may have specific KS activity,104 but clinical trials have generally not shown any such benefit of protease inhibitor-based cART in preventing or treating KS.103,105,106 The time to partial response or better with cART alone is commonly 6 to 12 months, and many responses are incomplete. In a randomized trial in South Africa of cART versus cART with early chemotherapy in treatment-naïve patients with largely T1 disease, the group randomized to chemotherapy showed a significantly higher KS response at 12 months (39% versus 66%), supporting a role for KS-specific therapy in patients with KS-associated morbidity. Overall, however, survival was similar in both arms.95 Patients infected with HIV occasionally demonstrate new KS or KS progression soon after cART initiation. It has been suggested that an immune reconstitution inflammatory syndrome may be contributing to the KS progression.107,108 However, the pathogenesis of worsening or initial development of KS in the setting of initiation of cART remains unclear. Such patients should be considered for additional anti-KS therapy.
Localized therapies are generally of limited utility in KS and can be associated with troublesome toxicity. However, for patients with symptomatic disease in a highly restricted area, a number of approaches have activity. While a biopsy is needed to confirm the diagnosis of KS, surgical resection is otherwise rarely indicated. Also, clear surgical margins do not suggest cure and KS can even develop in the site of a surgical scar.109 Topical 9-cis-retinoic acid (Panretin gel; Eisai Inc., Woodcliff Lake, NJ) is approved by the US Food and Drug Administration (FDA) for use in KS. While response in up to 45% of treated lesions are reported, local inflammation is common and the cosmetic result may also be inadequate.110–112 Intralesional injection or iontophoresis of low-dose vinblastine (0.1 mL of 0.1 mg/mL) or 3% sodium tetradodecyl sulfate injection (0.1 to 0.3 mL), as well as laser therapy and cryotherapy113 have also been described. Again, these local therapies can be painful, cosmetic results may be unsatisfactory, and disease progression outside of treatment site is common. Radiation therapy is effective, though progression outside the treated area is common. Local toxicities can include radiation dermatitis or ulceration. Over the longer term, induration and fibrosis may develop. For these reasons, it is generally reserved for disease that is quite limited, yet causing severe pain or distress. Dosing is individualized, ranging from an 8 Gy single dose to fractionated therapy to a total of 16 to 30 Gy.
Indications for systemic therapy in HIV-associated KS are imprecise and should be individualized. Strong indications include KS that is life threatening, symptomatic visceral or substantial pulmonary disease, and cutaneous disease that is extensive, ulcerating, or associated with edema or pain. In such cases, it should be initiated as soon as possible. Systemic therapy is also justified in more limited disease where response to cART alone is unlikely to be sufficient or timely from a symptomatic perspective, or where worsening KS would not be tolerated. It may also be justified when KS impairs quality of life due to social withdrawal or psychological distress. Although complete and long-lasting resolution of evident disease can be realized, this outcome does not imply cure. Treatment is aimed at decreasing tumor burden and symptom palliation, which may require chronic intermittent administration.
Several cytotoxic agents have activity in KS. The most commonly used agents are liposomal doxorubicin and paclitaxel. Liposomal doxorubicin is approved by the FDA for KS and has become the standard-of-care first-line chemotherapy, generally at the dose of 20 mg/m2 every 3 weeks.102 In the cART era, response rates of 45% to 80% may be anticipated.114,115 Liposomal daunorubicin is also approved for advanced KS, though less commonly used. An important consideration with anthracyclines is the risk of cumulative cardiotoxicity; the FDA warns against cumulative lifetime doses exceeding 550 mg/m2. While the risk appears to be lower with liposomal formulations than with bolus nonliposomal anthracyclines, this dose should not be exceeded without cardiac monitoring and individualized risk-benefit analysis. Paclitaxel is approved by the FDA as second-line therapy for KS, with responses ranging from 59% to 71% demonstrated in phase 2 trials conducted prior to the use of cART.116,117 A randomized trial comparing liposomal doxorubicin 20 mg/m2 every 3 weeks with paclitaxel 100 mg/m2 every 2 weeks in subjects with advanced KS showed comparable efficacy, but increased hematologic and neurologic toxicity in the paclitaxel arm.115 Symptomatic progression after ceasing therapy was common, and overall progression-free survival at 12 months was approximately 70%.115
Other agents with demonstrated KS activity include vincristine, vinblastine, doxorubicin, etoposide, and bleomycin. Oral etoposide 50 mg/day on days 1 through 7 of a 14-day cycle is active, with an overall response rate of 36% in previously treated patients, the majority of whom were not receiving cART.118 This approach may be useful in resource-limited settings, although the risk of secondary myeloid leukemia raises concerns with long-term administration. Early in the AIDS epidemic, high response rates were obtained with combination regimens of doxorubicin, bleomycin, and vinblastine or vincristine, although toxicity was often substantial.119 This regimen has been largely replaced by liposomal doxorubicin, which was shown in a randomized trial to be more effective and less toxic.120
A further systemic option is interferon (IFN)-α, a cytokine with immune modulatory and antiangiogenic activity. In HIV-associated KS, it is most active in patients with limited disease and preserved CD4 counts.121,122 Unfortunately, systemic side effects limit tolerability for many patients. Constitutional symptoms, cytopenias, mood disturbances including major depression, and hypothyroidism are common and may be severe. Most practitioners begin with IFN-α 1 to 5 × 106 units subcutaneous injection daily and gradually increase the dose as tolerated. IFN-α should be used in combination with cART in HIV-associated KS.123
A general strategy is to treat patients with KS until a response plateau or remission is attained. The number of cycles of chemotherapy required is variable. Patients with good immune reconstitution on cART are more likely to be able to stop chemotherapy after an early response, with maintained or even improved KS response with cART continuation. However, some patients require ongoing or periodic antitumor therapy in addition to cART. In cases of advanced immune suppression or uncontrolled HIV, KS may progress shortly after discontinuing therapy, and close observation is required. Growth of KS after therapy with a given chemotherapy does not necessarily imply tumor resistance, and a previously effective agent can often be used again.
There is a substantial unmet clinical need for novel, less toxic agents for KS. Established therapies have important limitations. In particular, there are few effective oral agents, and the most useful cytotoxic agents often are associated with neutropenia. The risk of cumulative anthracycline cardiotoxicity can limit use of liposomal anthracyclines, making them less favorable in heavily pretreated patients and patients with coexisting cardiovascular disease. Also, delivery of the most effective agents within existing health infrastructure is impracticable and/or unaffordable in resource-limited settings. Development of effective oral anti-KS agents could assist in addressing this significant global health problem.30,124
Oral agents that interfere with KSHV replication have been shown to prevent KS in patients with AIDS; a randomized clinical trial of ganciclovir for cytomegalovirus infections showed systemic administration was associated with a lower rate of KS development.125 However, the risk/benefit ratio of antiherpetic agents in preventing KS has not been evaluated and may be unfavorable. Furthermore, targeting KSHV replication with antiviral drugs has shown little utility in treating established KS. Ganiclovir and cidofovir have been evaluated in small studies, but not found to have meaningful activity in either classic or HIV-associated KS.126,127
Given the angiogenic nature of KS and its sensitivity to changes in immune status, both immune-modulating and antiangiogenic therapies have been explored in clinical trials. The cytokine IL-12 showed promising results alone and in combination with chemotherapy.128,129 Thalidomide, an oral agent with immune modulatory and antiangiogenic effects, has shown activity in two phase 2 studies, though its use is limited by toxicity.130,131 Studies are under way of thalidomide analogs. Targeted inhibition of angiogenesis with the monocolonal antibody to VEGF, bevacizumab,132 and imatinib,133 an inhibitor of c-kit and the platelet-derived growth factor receptor, have shown activity in phase 2 studies. Interestingly, in renal transplant–associated KS, modification of immunosuppression from cyclosporine to the mammalian target of rapamycin inhibitor sirolimus leads to tumor regression.134 However, in a study of sirolimus in HIV-associated KS, responses were limited and drug interactions with the antiretroviral drug ritonavir complicated dosing.135 Ongoing clinical studies will help define the role of additional novel agents in KS.
Kaposi Sarcoma Herpes Virus–Associated Multicentric Castleman Disease
The term Castleman disease is used to describe a group of related lymphoproliferative disorders. There are several forms of idiopathic Castleman disease that can be classified either anatomically (unicentric or multicentric) or by morphology (hyaline-vascular, plasma cell, or mixed histology). With the discovery of KSHV, it was recognized that this virus causes a plasmablastic variant of multicentric Castleman disease (MCD).136 Nearly all Castleman disease arising in the setting of HIV infection is KSHV-associated MCD (KSHV-MCD). This polyclonal137 hyperproliferative B-cell disorder is considered rare, although its incidence is not well defined. Unlike KS, KSHV-MCD may be more common since the advent of cART.138
KSHV-MCD is characterized by intermittent flares of inflammatory symptoms, including fevers, fatigue, cachexia, and edema, together with lymphadenopathy and splenomegaly. Gastrointestinal and respiratory symptoms are common, and rheumatologic, neurologic, and dermatologic manifestations may also be present. Laboratory abnormalities include anemia, thrombocytopenia, hypoalbuminemia, hyponatremia, and elevated C-reactive protein.139,140 The clinical course may wax and wane, but untreated, it is frequently fatal, with patients succumbing to inflammatory manifestations or progressing to large cell lymphoma.141
The symptoms of KSHV-MCD, while potentially severe, are nonspecific. KSHV-MCD should be considered in the differential diagnosis of patients with HIV and unexplained lymphadenopathy, splenomegaly, and/or inflammatory symptoms or autoimmune phenomena, particularly anemia or thrombocytopenia. Diagnosis generally requires excisional lymph node biopsy and demonstration of KSHV-infected plasmablasts. There is no validated staging or prognostic system, although definitions of symptomatic disease142 and National Cancer Institute (NCI) response criteria have been defined for clinical studies.139 These criteria rely on evaluation of clinical signs and common laboratory abnormalities. Symptomatic KSHV-MCD is associated with an elevated circulating KSHV viral load and associated cytokine dysregulation. In particular, viral IL-6 production and induction of human IL-6 and IL-10 are implicated as pathogenic factors.92,140,141 KSHV viral load decreases with effective therapy.139,140
There is no standard therapy for KSHV-MCD. However, based on an appreciation of the viral etiology and pathogenic mechanisms of KSHV-MCD, new therapeutic modalities are being developed that can control disease activity,101,139,142 improve overall survival,143 and may reduce the risk of KSHV-associated lymphoma.144 Best studied is the anti-CD20 monoclonal antibody rituximab.101,142 Most patients respond initially to rituximab-containing regimens. However, rituximab monotherapy may be insufficient in advanced disease, and it has been associated with exacerbation of concurrent KS. Approximately 30% of patients will relapse within 1 year.101,142 Rituximab combined with liposomal doxorubicin (in part to control KS) appears promising in preliminary reports.145 Zidovudine and ganciclovir are activated to toxic moieties by KSHV-encoded enzymes; based on this observation, high-dose zidovudine combined with ganciclovir has been tested and found to be active, although not as effective as rituximab in patients with severe inflammatory symptoms.139 Historically, chemotherapy regimens used in NHL141 and/or splenectomy141,146 have sometimes been used; however, recent studies suggest these can often be replaced by targeted approaches, sparing patients unnecessary toxicities and long-term infectious risks associated with splenectomy.147 Patients infected with HIV should receive cART, although intolerance to a number of drugs, including antiretrovirals, may occur until MCD is controlled. Given the many uncertainties in managing patients with KSHV-MCD, including length of treatment, role of maintenance therapy, and evaluation and management of concurrent malignancies, consideration should be given to referral to a clinical trial.
Inflammatory symptoms similar to those in KSHV-MCD have been described in patients infected with KSHV without pathologic evidence of KSHV-MCD.148 These patients presented with a constellation of fevers, cachexia, and laboratory abnormalities including cytopenias, hypoalbuminemia, and elevated C-reactive protein. Patients had elevated KSHV viral loads and disturbances of vIL-6 and human IL-6 and IL-10 similar to those seen in KSHV-MCD. However, splenomegaly, and lymphadenopathy were not prominent, and pathologic evidence of KSHV-MCD was not demonstrable. This syndrome has been provisionally named KSHV inflammatory cytokine syndrome, and a working case definition has been proposed.149 KSHV inflammatory cytokine syndrome may accompany KSHV-associated tumors (KS or PEL) or occur in their absence. Its pathophysiology, clinical outcomes, and relationship to KSHV-MCD are being evaluated.
LYMPHOID TUMORS IN PATIENTS WITH HIV
Overview
Lymphoid tumors (see Table 117.1) in individuals infected with HIV represent a range of histologies and include lymphomas seen in immunocompetent patients as well as lymphomas more specifically associated with HIV infection. Risk for individual tumors vary based on the underlying HIV disease status, while outcomes are largely defined by the natural history of a specific tumor as well as choice of an appropriate therapeutic approach for a given tumor. The most common tumors, DLBCL, BL, and cHL, generally occur among patients infected with HIV with relatively preserved immune function. Treatment options for these tumors are informed by clinical studies, and they are highly curable, highlighting the importance of accurate diagnosis of HIV disease status. Less common tumors include PCNSL, systemic DLBCL with immunoblastic phenotype, plasmablastic lymphoma, PEL, and KSHV-MCD (previously discussed with KS). These tumors continue to represent a clinical challenge due to lack of prospective clinical studies, but should also generally be approached with curative intent.
Prior to the cART era, prognosis for patients with HIV with lymphoid tumors was determined primarily by CD4 cell count.150 Systemic ARLs were often treated as a single disease entity, often with reduced dose chemotherapy regimens, and outcomes were generally poor. However, clinical trials performed in the cART era demonstrate that standard-dose chemotherapy regimens can be administered and improve survival. While baseline CD4 cell count remains important prognostically, lymphoma-specific features49,52,55,151 and treatment related factors are now critical, especially in patients with CD4 cell counts >100/mm3.
Clinical decision making must be based both on lymphoma classification and assessment of the immune status and possible AIDS-related comorbidities. The clinical status of patients with HIV infection ranges from healthy with preserved immunity to immunosuppressed with multiple comorbidies. An individualized clinical approach must take this potential spectrum into account. For healthier patients, treatment is similar to the HIV-unrelated counterpart. However, patients with advanced AIDS may not tolerate therapy as well, and close coordination of chemotherapy with HIV management and supportive care is critical. Importantly, poor performance status is often due to the underlying malignancy or treatable opportunistic infections, and even patients with poor performance status should be considered for full-dose curative therapies in most instances. With a shift in demographics of HIV infection to communities with less access to medical care, patients continue to present with lymphoma as the initial manifestation of HIV. These cART-naïve patients usually have HIV that can be rapidly suppressed, and with effective lymphoma treatment and access to cART, they generally have favorable long-term survival. Effective cancer therapy in these patients is therefore essential.
Pathophysiology
HIV-related lymphomagenesis depends on numerous factors, including the degree of immune suppression (discussed with each specific tumor type) and cumulative time with uncontrolled HIV viremia.47 The pathogenesis is related to B-cell immune activation. Elevated serum cytokines including IL-6, IL-10,42,152 and tumor necrosis factor-β, as well as the activation marker, soluble CD30,153 are detectable prior to ARL diagnosis. Several genetic polymorphisms related to B-cell activation are also associated with ARL risk, including the 3′A variant of stromal cell–derived factor 1154 and 592C/C IL-10 promoter.42 The HIV coreceptor CCR5 deletion variant CCR5-Δ32 also confers protection against NHL, independent of its HIV protective effect.155 In the setting of HIV, B-cell activation in EBV-infected cells may be increased in part due to expression of a virally encoded CD40-like latent membrane protein.156 Activation also occurs also in EBV-uninfected B-cells through other inflammatory stimuli44,48 and perhaps direct interactions with HIV itself.157,158
Regardless of the nature of immune activation, translocations and aberrant somatic hypermutation are common features of many ARL,159 including DLBCL and BL. For most ARL, B-cell activation leads to upregulation of activation-induced cytidine deaminase,160 an enzyme required for GC class switch recombination and somatic hypermutation. However, activation-induced cytidine deaminase also can induce mutations and pathogenic translocations,161 which is a critical step in lymphomagenesis. Translocation of c-myc/Ig are seen in not only in BL, but are also present in DLBCL in patients infected with HIV at a higher frequency than observed in HIV-uninfected cases,162 and a majority of EBV+ plasmablastic lymphomas.163
Clinical Presentation, Staging, and Approach to Treatment
Lymphoid malignancy can be the initial presenting illness in HIV,164,165 and HIV testing should be performed in patients with aggressive B-cell lymphomas or cHL. ARL frequently presents with either a rapidly growing mass or “B” symptoms (fever, night sweats, or weight loss in excess of 10% of the normal body weight). Extranodal involvement of bone marrow, gastrointestinal tract, and central nervous system (CNS) is common.37 Staging should include computed tomography of neck, chest, abdomen, and pelvis; fluorodeoxyglucose (18FDG)-positron emission tomography (PET); lactate dehydrogenase; complete blood count with differential; bone marrow biopsy; and lumbar puncture with evaluation of cerebral spinal fluid (CSF) by cytology and flow cytometry. Evaluation of cardiac function is recommended. CNS imaging, generally with magnetic resonance imaging (MRI) with gadolinium, should be performed to evaluate for CNS involvement in patients with NHL, particularly BL. CNS imaging is not standard for cHL or KSHV-MCD, although in rare cases, cHL in patients with HIV can involve the CNS. Baseline HIV viral load, CD4 count, HBV core antibody and surface antigen, and hepatitis C virus serology should be evaluated. Patients with detectable HBV core antibody or surface antigen should be screened for quantitative HBV viral load.
A high percentage (12% to 57%) of patients with ARL, especially BL, have CNS involvement at presentation.37,54,55 Routine CNS prophylaxis is generally used, although some favor stratifying patients based on extranodal disease. Prophylaxis has varied between studies, but generally includes intrathecal methotrexate (12 mg) and/or cytosine arabinoside (50 mg) for a total of four to eight doses. CNS involvement by DLBCL confers a poor prognosis. Several intensive intraventricular and intrathecal methotrexate schedules have shown activity. Most commonly, therapy is administered using an Ommaya reservoir twice weekly until 2 weeks after negative CSF flow cytometry is documented (for a minimum of eight doses), and then continued weekly for 6 to 8 weeks, then monthly for 6 months.55,56
Supportive care and use of cART during lymphoma therapy deserves special consideration. While effective cART has reduced the incidence of HIV-associated lymphoid tumors and has improved survival, no randomized trial exists to clarify the utility of cART during chemotherapy. On one hand, earlier control of HIV viremia and immune reconstitution may improve long-term infectious outcomes, especially in patients with low CD4 counts. However, there are important potential pharmacokinetic and toxicity interactions between cART and chemotherapeutic agents. As a class, protease inhibitors (especially ritonavir) inhibit CYP3A4 and have potential interactions with other drugs, such as cyclophosphamide.166–168 Additionally, zidovudine should generally be avoided because of additive hematotoxicity, and renal function should be monitored in patients on tenofovir. Nonetheless, substantial data from phase 2 studies that have included cART suggest that chemotherapy and cART can be concomitantly administered,169 usually with growth factor support. However, use of cART during chemotherapy for DLBCL and BL is not essential, and phase 2 studies of dose-adjusted etoposide, doxorubicin, and vincristine with oral prednisone and bolus cyclophosphamide (DA-EPOCH; both with and without rituximab) demonstrated excellent results while withholding cART during lymphoma treatment.54,55 More recently, pooled data from 19 trials with varied approaches to cART suggests that after correction for CD4 count and other prognostic and treatment-related factors, concurrent cART may be associated with improved response rates and a trend toward improved overall survival.170 While these studies suggest an advantage for concurrent cART, it is important that in the face of toxicity-limiting ability to administer curative-intent therapy, suspension of nonessential drugs, including cART, is a reasonable first maneuver, especially in patients with CD4 counts >100 cells/mm3. When cART is discontinued, it should be resumed promptly after completion of therapy. Often, physicians who treat patients infected with HIV who have lymphoid tumors, continue cART, preferably with a protease inhibitor–sparing regimen. Additionally, newer antiretroviral drugs to avoid during chemotherapy are class-combination formulations that contain potent pharmacologic boosters such as cobicistat, which may have substantial effects on the chemotherapy. It is also reasonable to defer cART initiation in patients not on therapy until after initiating chemotherapy.
Given risk of infections both during and after completion of chemotherapy, especially in patients with CD4 counts <100 cells/mm3, attention to supportive care is critically important. All patients infected with HIV being treated for lymphoma, regardless of baseline CD4 count, should receive prophylaxis against PCP, preferably with trimethoprim-sulfamethoxazole. Patients with a CD4 count <50 to 100 cells/mm3 also require azithromycin 1,200 mg weekly as prophylaxis against atypical mycobacterial infections. Prophylaxis against herpes simplex virus reactivation using valacyclovir should be strongly considered. Patients with detectable hepatitis B viremia require antiviral therapy for HBV. Care is required to ensure that therapy of intercurrent HBV avoids compromising HIV control, as single-agent therapy for HBV will increase the likelihood of a specific HIV mutation, M184V, which renders patients resistant to several important antiretroviral agents. Patients with mucosal Candida infections should not receive azoles concurrently with chemotherapy.
Diffuse Large B-Cell Lymphoma
The majority of NHL occurring in patients with HIV are large B-cell lymphomas or BL. With more sophisticated molecular categorization, DLBCL itself is increasingly recognized as a constellation of genetically unique diseases. In the setting of HIV, various large B-cell lymphoma subtypes are associated with the degree of immune suppression and in some cases dysregulated control of oncogenic herpesviruses.
Immunohistochemical (IHC) markers are helpful in categorizing DLBCL subtypes (Table 117.4) and may provide important diagnostic and prognostic information.57,171–173 However, DLBCL seen in HIV is often categorized as DLBCL, not otherwise specified, because it does not meet the criteria of the other more highly specified DLBCL types. Although many cases are DLBCL, not otherwise specified, phenotypic characterization as centroblastic (associated with higher CD4 cells and with GC phenotype) and the immunoblastic variant (associated with lower CD4 cells and the ABC phenotype) remains useful.55 Gene expression profiling (GEP) is the gold standard for distinguishing GC from ABC DLBCL in patients not infected with HIV. However, gene expression profiling and associated IHC algorithms were not developed or validated in the setting of HIV-associated DLBCL. Identification of DLBCL subtypes by ICH does not consistently segregate the biologic entities into the GEP-defined prognostic groups, regardless of HIV status.174 However, assessment of GEP in HIV DLBCL is needed to insure that there are no major differences in ABC and GBC DLBCL subtypes based on HIV status. A combination of IHC for B-cell lineage markers and the KSHV protein, latency-associated nuclear antigen, in situ hydridization for EBV-encoded small RNAs, and evaluation for c-myc/Ig translocations is warranted, and is generally necessary to categorize the rarer large B-cell lymphoma subtypes such as PBL and PEL. Determination of B-cell clonality by polymerase chain reaction (PCR) evaluation of immunoglobulin genes is sometime helpful, and additional molecular diagnostic biomarkers are likely to become increasingly important. Given development of novel agents such as lenalidomide and ibrutinib in HIV-unrelated DLBCL, it will become increasingly important to determine DLBCL subtypes in the setting of HIV, as these agents appear particularly active in ABC DLBCL. In anticipation of these advances, the NCI-sponsored AIDS-Malignancy Consortium (AMC) is conducting trials to determine safety and dosing considerations of novel agents in conjunction with the various classes of cART.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








