The multistep model for breast carcinogenesis suggests that invasive carcinomas arise from preinvasive “hyperplastic” and neoplastic proliferations. These early proliferative lesions have taken on greater significance as a result of the mammographic screening and detection programs. Pathologists are encountering these proliferative lesions with increasing frequency, and this has highlighted deficiencies in classification systems as well as a lack of data on natural history, making clinical management a challenge.
Invasive carcinomas are divided into ductal carcinoma no-special-type (IDC-NST) and the “special types,” of which lobular carcinoma (ILC) is the major component. While there has been little debate or controversy about the precursor nature of ductal carcinoma in situ (DCIS), there is considerable argument regarding the role of lobular carcinoma in situ (LCIS) in progression to invasive disease. The picture is further complicated by the identification of lesions that are qualitatively the same but less developed (atypical lobular hyperplasia, ALH) and those showing intermediate features between hyperplasia and in situ carcinoma (atypical ductal hyperplasia, ADH).
The evidence for progression and the risks associated with these lesions is derived from morphology, clinical follow-up, and molecular relationships. The path from normal to invasive carcinoma is certainly not a linear one, and there is evidence that breast cancer and the evolutionary pathways are heterogeneous.1 The overall paucity of data to stratify individual patients into meaningful risk categories and the often long time-frame to progression has created challenges in surgical management of these preinvasive lesions. This is further confounded by the diagnostic difficulties from small tissue samples in core biopsies used as part of the workup in screening programs. In this chapter, we discuss the pathology, biology, and management of lobular neoplasia and atypical ductal hyperplasia.
Foote and Stewart gave the first clear clinical-pathologic description of LCIS in 19412 describing the morphologic similarities between these in situ lesions and ILC. This, together with the frequent concurrent diagnosis of LCIS and ILC, suggested a progressive relationship prompting management, ultimately removal of LCIS by mastectomy. This management strategy was subsequently supported by independent clinical follow-up that demonstrated a cumulative (and bilateral) risk of carcinoma in LCIS patients.3 Over the next 30 years, the combination of bilateral risk, the long time-frame to progression, and the identification of IDC in association with LCIS raised questions about the precursor nature of LCIS. The lesion was considered only a “risk indicator,” and hence management recommendations became varied, ranging from mastectomy to surveillance only.4-10
ALH was introduced more recently to describe lesions that are less well-developed but morphologically similar to LCIS. In 1978 the term “lobular neoplasia” (LN) was introduced to encompass both ALH and LCIS4; however, the term has not gained universal acceptance.
Lack of specific clinical abnormalities has made estimation of the true incidence of LN very difficult. LN is neither palpable nor usually associated with mammographic abnormalities,11-13 nor has it any guiding macroscopic features for the pathologist sampling the specimen. As a result, diagnosis is often an incidental microscopic finding in breast biopsies taken for other reasons. Hence the prevalence of ALH and LCIS is likely to be underestimated in the general population. The incidence of LCIS in otherwise benign breast biopsy is reported as between 0.5% and 3.8%.4-6,14-18 Compared to both DCIS and ILC, LCIS is more frequently diagnosed in younger, premenopausal women, usually between 40 and 50 years of age.5,6,11,15,19
LCIS is characteristically multifocal and bilateral, with approximately 50% demonstrating multiple foci in the ipsilateral breast and 30% in the contralateral breast.4,5,18,20-23 The multifocality of these lesions suggests an underlying genetic predisposition. There are data to link rare cases with germ-line mutations in the E-cadherin gene, but this accounts for a very small number of cases.24 Compared to the general population, ALH has a 4- to 5-fold and LCIS an 8- to 12-fold greater lifetime risk, respectively, of developing invasive carcinoma.16,19,25 This translates to approximately 1% per year. Furthermore, the risk of developing a contralateral invasive cancer is greater in patients with LCIS compared to DCIS.26-29
However, despite the bilateral risk associated with lobular neoplasias, the risk is still skewed toward the ipsilateral breast.16,17,30 The reported time between LCIS diagnosis and the development of invasive carcinoma ranges from 15 to 30 years.5,18 Seventy percent of invasive carcinomas reported subsequent to LCIS diagnosis are of the lobular type5; conversely, LCIS has been identified in up to 91% of ILC cases.31 Wheeler and associates found that although the risk of ILC is 18-fold higher in association with LCIS, IDC is also found in association with LCIS at a 3 to 4 times higher rate than the general population.6 IDC, found subsequent to LCIS diagnosis, is thought to be due to the coexistence of DCIS, at least in a proportion of cases.32,33
Figure 17-1A and B show examples of ALH and classic LCIS. While overall lobular architecture is maintained in LCIS, the acini are full and distended with a monomorphic population of small loosely cohesive cells. The cells are round, polygonal, or cuboidal and have a high nuclear-to-cytoplasmic ratio. The cells contain intracytoplasmic lumina or magenta bodies. The uniform nuclei contain fine and evenly dispersed chromatin. Mitoses, calcification, or necrosis are rarely observed. The differentiation between ALH and LCIS is rather arbitrary and quantitative rather than qualitative. In ALH the acini are not completely distended and residual lumina may be present. The cells comprising these “classic” ALH and LCIS lesions are also known as type A cells, which contrast with type B cells, which have larger vesicular nuclei.34
Figure 17-1
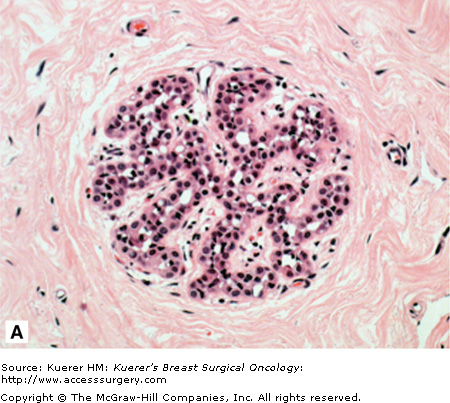
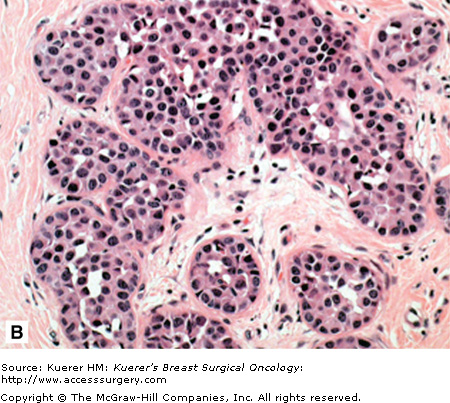
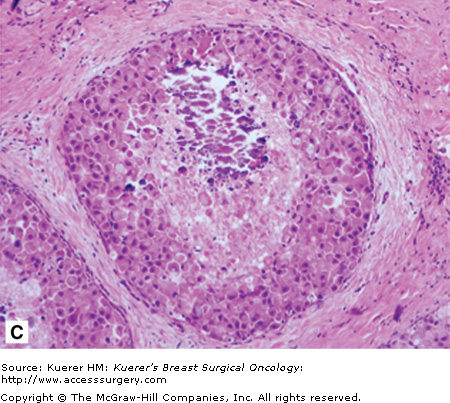
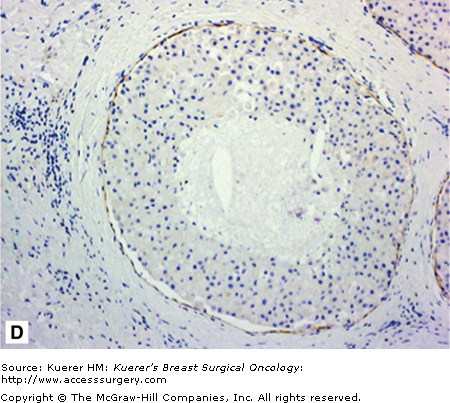
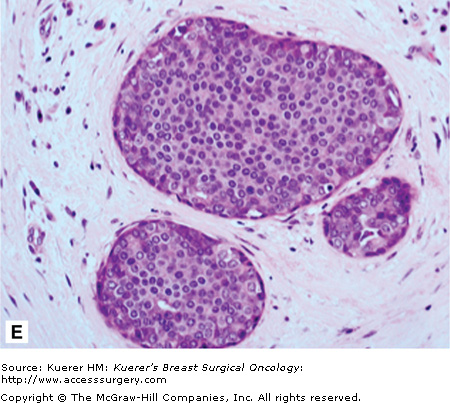
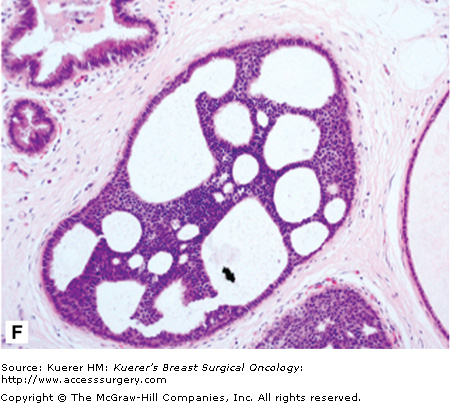
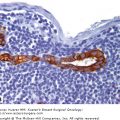
A. Atypical lobular hyperplasia—minimal distension of lobular unit with cells similar to those seen in lobular carcinoma in situ. B. Lobular carcinoma in situ—small monomorphic cells distending the terminal duct lobular unit, with no necrosis or mitoses. C. Pleomorphic variant of lobular carcinoma in situ—the cells are bigger with more pleomorphism, there is central comedo-type necrosis with calcification, and hence the lesion is mistaken radiologically and pathologically for high-grade ductal carcinoma in situ. E-cadherin is negative and illustrated in (D)E. The lesion depicted is difficult to classify; it is a solid low-grade proliferation that could be a low-grade DCIS of solid type or could also be LCIS. E-cadherin staining is useful in such cases to help classify the lesion. F. Atypical ductal hyperplasia. The lesion resembles low-grade DCIS but there is a retension of normal epithelium in part of the duct.
Pleomorphic lobular carcinoma in situ (PLCIS) is a recently recognized variant in which there is greater nuclear polymorphism (Fig. 17-1C), prominent nucleoli, more abundant and eosinophilic cytoplasm, and apocrine differentiation.1,35-37 Unlike classic LCIS, necrosis and calcification can be found in PLCIS (which can be detected by mammography as suspicious for DCIS) and is associated with the cytologically similar pleomorphic invasive lobular carcinoma (PILC).
One of the most difficult but important differential diagnoses for classic LCIS is the low-grade, solid form of DCIS (Fig. 17-1E). PLCIS is mistaken radiologically and histopathologically for high-grade DCIS, which has different management implications for the patient. Distinguishing LCIS from low-grade solid DCIS is particularly challenging, especially when the DCIS involves the acini (cancerization of the lobules).32,38,39 Secondary lumen formation, cellular cohesion, and greater nuclear size and pleomorphism all point to a DCIS diagnosis. Immunohistochemistry for E-cadherin can be valuable in distinguishing the lesions (Fig. 17-1D).Concurrent involvement of the lobules with LCIS and DCIS does occur, and in such instances both are recorded and the patient managed for DCIS. Cases in which LCIS arises within another type of breast lesion, such as sclerosing adenosis or radial scar, can be mistaken for invasive carcinoma. The utilization of ancillary techniques such an immunohistochemistry can be invaluable here to detect and demonstrate the integrity of the myoepithelial layer or basement membrane.
Due to the difficulty in classification and assigning risks based on morphology, there has been a hope that molecular subtyping would provide a better means of managing patients with these early preinvasive lesions. To date, there are no molecular data that help to identify lesions that will recur or progress and hence need more aggressive therapy.
Over 90% of LCIS cases are immunopositive for the estrogen receptor (ER) and progesterone receptor (PgR) and do not exhibit other classic biomarkers of aggressiveness such as HER2 overexpression, p53, and/or high Ki67 index.40-45 This immunophenotype is very similar to both ILC and low-grade DCIS/IDC, but contrasting with high-grade DCIS/IDC. Despite the higher grade, PLCIS is also usually ER and PgR positive, but more frequently shows aggressive features such as HER2 overexpression, p53 positivity, and a higher Ki67 proliferative index. A higher frequency of gross cystic disease fluid protein-15 (GCDFP-15) has also been observed in pleomorphic variants, consistent with the observation of apocrine differentiation.
One of the most distinguishing molecular features of LN is the lack of E-cadherin expression in the vast majority of cases compared to ductal lesions,7,36,38,46-56 hence its use in clinical practice to classify these proliferations. Loss of this cell–cell adhesion protein is thought to account for the characteristic discohesive morphology of lobular neoplastic cells. E-cadherin down regulation in LN has been found to occur by a variety of mechanisms, including the frequent loss of chromosome 16q or the more specific loss of heterozygosity of the E-cadherin locus, which is usually accompanied by truncating mutations or promoter methylation of the other allele. Reports of the same E-cadherin mutation in both LCIS and adjacent ILC has been the most direct and important evidence for the precursor role of LCIS.49 Interestingly, although E-cadherin down regulation is observed in ALH, one paper suggests that mutations in the gene are rare, suggesting that the mutations occur after initial loss of expression.48 Further work is required to fully understand the mechanism of LN E-cadherin loss, which may indeed be driving the tumourigenesis.
Array Comparative Genomic Hybridization (aCGH) studies that examine global genetic change further confirm that loss of 16q is a consistent and early event in the development of lobular neoplasms. The other most frequent chromosomal changes observed in LCIS include losses of 8p, 16p, 17p, 17q, and 22q, and gain of 1q and 6q. There are no significant differences in genetic alterations between ALH and LCIS, which strongly demonstrates their close relationship.57 Interestingly there is significant overlap with changes occurring in low-grade DCIS, which suggests a close evolutionary development of lobular and low-grade ductal pathways.56,58
Comparison with ILC demonstrates strong concordance of genetic changes, further supporting the hypothesis of a common clonality of these lesions and suggesting that LCIS is a direct precursor to ILC.59,60 PLCIS is also genetically similar to LCIS, demonstrating a close relationship, but also exhibits additional changes such as amplification of oncogenic loci such as MYC (8q24) and HER2 (17q12), which again suggests it is a more aggressive lesion.36,58,61 Although of significant benefit in demonstrating the progressive relationship between these lesions, these studies have yet to provide us with new prognostic markers that assist in clinical decision-making regarding the likelihood of progression of preinvasive lesions to invasive carcinoma.
Atypical ductal hyperplasia (ADH) is a very specific lesion that has cytomorphologic features both intermediate and overlapping with hyperplasia of usual type and low-grade DCIS.62 Size criteria have also been introduced.16,63 An example of ADH is shown in Figure 17-1F.
ADH is reported in 4% of all symptomatic benign biopsies64 and imparts an increased risk of invasive breast cancer of 4- to 5-fold.65,66 The reported increase in risk to 11-fold in patients with a family history of breast cancer67 has recently been challenged by a new Mayo Clinic study68 suggesting that the phenotype is influenced by both inherited risk and lifetime exposures. In a study by Page and associates, 12% of cases developed invasive carcinoma at an average of 16 years postdiagnosis.16 Including DCIS as a cancer event, the Mayo Clinic recently found as many as 20% of ADH patients progressed to cancer within approximately 13 years, and this within the ipsilateral breast68 as had already been shown for untreated DCIS.69
ADH (Fig. 17-1F) bears high morphologic similarity to low-grade DCIS (Fig. 17-1E). It is composed of regularly arranged monotonous round, cuboidal, or polygonal cells, enclosed by a basement membrane. Mitoses are rare and nuclei are evenly distributed. Several different growth patterns have been observed, including cribriform and micropapillary patterns. Small necrotic foci may also be seen within ADH lesions. The proliferation involves part of the duct space and the secondary lumina are punched out as well as irregular. While most bridges are solid, occasional streaming of cells is also seen. Hence the features are intermediate between hyperplasia and DCIS. A size cut-off of 2 or 3 mm has also been introduced in the definition.
Intraobserver concordance for ADH diagnosis is notoriously poor,70,71 although the work of Page, Dupont, and colleagues has done much to improve agreement amongst observers. Their methods emphasise the importance of using 3 different sets of criteria: histologic pattern, cytologic features, and anatomic extent of lesion,16,62,72 which involves observation at both high- and low-power magnification for the features listed above.
Expression of specific molecular markers in precursor and preinvasive lesions has been studied by multiple groups (reviewed in Zagouri et al73). As with LN and low-grade DCIS, ADH is uniformly positive for ER and PgR. HER2 is generally not overexpressed in the lesions.74 HER2 amplification has been detected in a small proportion of ADH,75 although there are conflicting reports as to whether it imparts greater risk of developing invasive carcinoma.42,76 The well-differentiated features of ADH are further confirmed on a molecular level by low Ki-67 expression (a proliferative marker), bcl-2 positivity (a proapoptotic marker negatively correlating with aggressiveness77), and p53 negativity.78 Despite these findings, no molecular markers have been found to have independent prognostic significance for ADH, indicating that more research is needed in this area.
As for lobular neoplasia, the advent of microarray technology has provided further evidence for the model of progression through ADH to DCIS and IDC. Gene expression profiling of these lesions (including multiple matched samples from the same patient) and comparison with normal breast tissue demonstrate that most alterations that distinguish invasive carcinoma from normal tissue are present in ADH.79 This supports the notion of a clonal relationship between the distinct pathologic stages and is further supported by subsequent principal component analysis of the data.80,81 The same study found that gene expression patterns correlated highly with grade, that is, there was more difference between samples of different grades (high or low) than between samples of different stages of progression. Notably, all ADH samples demonstrated a grade I molecular profile and clustered with low-grade DCIS and IDC.79
Molecular genetic analysis is consistent with the morphologic similarities between ADH and low-grade DCIS (reviewed in Reis-Filho and Lakhani82). Although there are reports of ADH with no genetic change by CGH analysis,83 others have shown recurrent loss of 16q and 17p,84 which corroborates LOH studies of matched pairs of ADH and DCIS.85,86 One study demonstrated that 45% of ADH lesions shared at least 1 LOH with adjacent IDC.87 While this is very strong evidence for ADH as a precursor to DCIS in breast cancer progression, it has not, as yet, resulted in new methods for identifying those ADH lesions that will progress to invasive cancer and those that will not.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree