HER2 Inhibitors
Sara M. Tolaney
Eric P. Winer
Ian E. Krop
HER2/neu is a member of the erbB family of transmembrane tyrosine kinase receptors. This family includes the epidermal growth factor receptor (EGFR), HER2, HER3, and HER4. These receptors regulate cell growth, differentiation, and survival. Amplification of the HER2 gene occurs in 20% to 25% of breast cancers and is associated with poorly differentiated, high-grade tumors, resistance to therapy, higher rates of recurrence, and a higher incidence of brain metastasis.1,2
Trastuzumab, a recombinant humanized monoclonal antibody against the extracellular domain (ECD) of HER2, has made a major impact on the treatment of HER2-positive breast cancer. Clinical studies demonstrate that trastuzumab can improve disease-free and overall survival in HER2-positive patients in both the adjuvant and advanced disease settings.3,4
In the adjuvant setting, addition of weekly trastuzumab for 52 weeks to chemotherapy with doxorubicin/cyclophosphamide followed by paclitaxel in women with resected HER2-positive breast cancer reduced relapse rates by 50% (3-year disease-free survival was 87% with trastuzumab and 75% without it) and improved survival by 33% (94% with trastuzumab and 91% without it).4
Although response rates of HER2-positive metastatic breast cancer to trastuzumab monotherapy are low, ranging from 11% to 34%,5,6 when combined with chemotherapy, such as a taxane, response rates approach 70% in the first-line setting.3,7 In the pivotal trial in which women with previously untreated HER2-positive metastatic breast cancer were randomly assigned to paclitaxel or doxorubicin/cyclophosphamide versus the same chemotherapy with trastuzumab, the addition of trastuzumab increased time to progression from 4.6 to 7.4 months.8 Subsequent studies have demonstrated that in addition to the taxanes, trastuzumab acts synergistically with a large number of other chemotherapeutic agents including vinorelbine, capecitabine, gemcitabine, and the platinum agents.7,9, 10, 11, 12, 13
While trastuzumab therapy is effective in combination with chemotherapy in improving response rates and disease-free survival, the majority of patients with metastatic disease develop disease progression within 12 months. Interestingly though, patients who develop disease progression on one trastuzumab-containing chemotherapy regimen can still benefit from continuation of trastuzumab with another chemotherapy agent. In a trial in which patients with HER2-positive metastatic breast cancer that had progressed through one line of trastuzumab-based therapy were randomly assigned to capecitabine with or without trastuzumab, continuation of trastuzumab with capecitabine led to a doubling of the response rate compared to capecitabine alone, and was associated with an improvement in time to progression from 5.6 to 8.2 months.14 Furthermore, in a phase III trial for patients with HER2-positive metastatic breast cancer who were heavily pretreated with an anthracycline, a taxane, and trastuzumab, patients were randomly assigned to lapatinib or lapatinib in combination with trastuzumab.15 A significant improvement in progression-free survival was observed with the addition of trastuzumab (8.1 to 12.0 weeks).
Despite some patients having the ability to respond to trastuzumab even after having progressed through another trastuzumab-based regimen, patients will almost inevitably experience tumor progression. Therefore, the need for additional therapies directed against HER2-positive breast cancer is critical.
Development of Trastuzumab
HER2 encodes a 185-kDa transmembrane tyrosine kinase that was first identified as an oncogene activated by a point mutation in chemically induced rat neuroblastomas. Although mutations in human HER2 appear to be quite rare,16 the gene is amplified in a subset of human breast cancers, leading to marked overexpression of the HER2 protein. In the 1980s, interest grew to pursue development of a monoclonal antibody against HER2 and a large array of monoclonal antibodies were screened in proliferation assays against normal and tumor cells that expressed a broad spectrum of HER2 levels. Overall, the best in vitro inhibitor of growth of HER2-overexpressing cells was the monoclonal 4D5 (muMAb4D5). In addition to its in vitro effects, when this antibody was administered to mice with human breast cancer xenografts that overexpressed HER2, it suppressed tumor growth.17,18 Given these results, it was postulated that this antibody may be a useful therapeutic agent.19 Because the human immune system would recognize 4D5 as foreign and develop neutralizing antibodies to it, a humanized form of the monoclonal antibody was developed. A humanized version of muMAb4D5 (rhuMAb HER2) was engineered to retain the binding specificity of the parent antibody and also encode human-like residues in the hypervariable complementarity determining regions (Fig. 28-1). The humanized antibody is significantly less antigenic than the murine antibody and binds to the HER2 ECD with an affinity three times greater than the murine antibody.20
Pharmacokinetics of Trastuzumab
The pharmacokinetics of trastuzumab are not completely defined (Table 28-1). Initial work suggested that the pharmacokinetics were
dose-dependent,6 with half-lives of 2.7, 3.1, 8.8, and 10.4 days reported after single doses of 1, 2, 4, and 8 mg/kg, respectively.21 Subsequently, trastuzumab has been found not to exhibit dose-related nonlinear pharmacokinetics. A retrospective analysis of pharmacokinetic data for weekly trastuzumab using a two-compartment model indicated that the half-life of trastuzumab is 28.5 days.22 These data provided the rationale for evaluating a higher trastuzumab dose at a longer dosing interval and subsequent results demonstrated that trastuzumab 6 mg/kg every 3 weeks produced the same plasma trough levels as trastuzumab 2 mg/kg weekly. A phase II study of trastuzumab administered every 3 weeks demonstrated that this schedule did not compromise the efficacy and safety of trastuzumab in patients with metastatic breast cancer and that the average exposure was similar to that observed with weekly treatment.23
dose-dependent,6 with half-lives of 2.7, 3.1, 8.8, and 10.4 days reported after single doses of 1, 2, 4, and 8 mg/kg, respectively.21 Subsequently, trastuzumab has been found not to exhibit dose-related nonlinear pharmacokinetics. A retrospective analysis of pharmacokinetic data for weekly trastuzumab using a two-compartment model indicated that the half-life of trastuzumab is 28.5 days.22 These data provided the rationale for evaluating a higher trastuzumab dose at a longer dosing interval and subsequent results demonstrated that trastuzumab 6 mg/kg every 3 weeks produced the same plasma trough levels as trastuzumab 2 mg/kg weekly. A phase II study of trastuzumab administered every 3 weeks demonstrated that this schedule did not compromise the efficacy and safety of trastuzumab in patients with metastatic breast cancer and that the average exposure was similar to that observed with weekly treatment.23
 FIGURE 28-1 Diagram of HER2 and Trastuzumab (Herceptin) Fab complex. (Reprinted from Cho HS, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 in complex with the herceptin Fab. Nature 2003;421:757, with permission.) (Please see Color Insert.) |
TABLE 28.1 Key features of trastuzumab | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
The route of elimination is also not well defined, but it appears to depend on high serum levels of circulating HER2 ECDs shed from breast tumor cells. Patients with high baseline levels of ECDs are more likely to have a lower trough concentration of trastuzumab in the serum. No dose adjustments are necessary for renal dysfunction or age.
Managing Toxicity
Trastuzumab is well tolerated, with the most common adverse events being infusion-related reactions. Approximately 40% of patients will experience a reaction during the initial infusion, primarily fevers and/or chills.24 Other infusion-related reactions that have been observed include nausea, vomiting, pain at the tumor site, rigors, headache, dizziness, dyspnea, hypotension, rash, and asthenia. Usually these reactions are mild in nature and do not require discontinuation of the drug. Often, treatment with acetaminophen, diphenhydramine, or meperidine may be used. In addition, the subsequent dose should be infused over 90 minutes, but if symptoms subside, future infusions can be given over 30 minutes.
The most significant safety concern related to trastuzumab is the potential for cardiac toxicity, which occurs in 2% to 4% of patients receiving trastuzumab. The mechanism of trastuzumab-related cardiotoxicity is unknown, but is thought to be related to expression of HER2 on cardiac myocytes and potentially to suppression of the HER2-mediated myocyte stress response.25 Trastuzumab has the potential to induce congestive heart failure, and rates appear to be significantly higher when trastuzumab is administered at the same time as an anthracycline, with an incidence as high as 28%.3 Administration of trastuzumab concurrently with an anthracycline agent is therefore not recommended. Patients receiving trastuzumab should undergo evaluation of their ejection fraction before initiation of therapy, and frequent reevaluation (approximately every 3 months) is necessary, with early discontinuation of the drug if congestive heart failure or a drop in the ejection fraction occurs.
Other serious adverse events from trastuzumab are very rare. Pneumonitis, adult respiratory distress syndrome, pulmonary fibrosis, and death have been infrequently reported, but most fatal events seem to occur in patients with preexisting pulmonary dysfunction. Patients with severe underlying pulmonary disease should be treated with caution.
Mechanism of Action of Trastuzumab
In order to understand how trastuzumab resistance might develop, it is important to first understand the mechanism by which trastuzumab induces tumor regression. While the exact mechanism of action of trastuzumab remains unclear, several mechanisms have been proposed. HER2 is able to modulate cell function by activating several important cell signaling pathways, including the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) cascades. By binding to HER2, trastuzumab inhibits signaling via these pathways, and promotes cell cycle arrest and apoptosis (Fig. 28-2). This hypothesis is supported by in vitro data demonstrating that trastuzumab inhibits PI3K and its target Akt in breast cancer cells overexpressing HER2, but not in cell lines that do not exhibit HER2 amplification.26
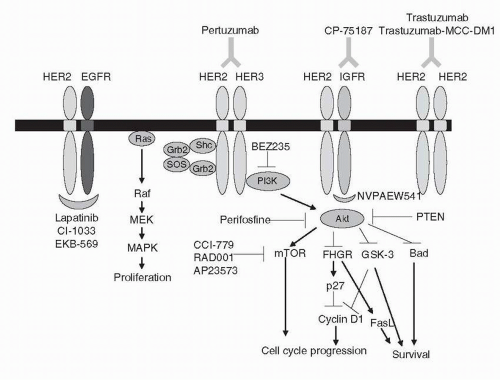 FIGURE 28-2 The HER2 signaling pathway with novel targeted agents. Ligand binding induces dimerization and activation of the intracellular tyrosine kinase, leading to activation of key downstream effectors. FasL, Fas ligand; FKHR, forkhead homologue in rhabdomyosarcoma; Grb, growth factor receptor-bound protein; GSK, glycogen synthase kinase; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; mTOR, molecular target of rapamycin; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homologue; SOS, son-of-sevenless guanine nucleotide exchange factor. (Please see Color Insert.) |
HER2-positive cancer cell lines treated with trastuzumab in vitro undergo arrest during the G1 phase of the cell cycle. This is postulated to be due to a decrease in the expression of proteins that sequester the cyclin E/Cdk2 inhibitor protein p27.27 Trastuzumab treatment therefore leads to release of p27, allowing binding and inhibition of cyclin E and Cdk2 complexes. Since entry of cells into S phase depends on the activation of these cyclin-dependent kinases, cells are not able to progress from G1 to S phase. This suggests that HER2-overexpressing tumors depend on maintenance of p27 sequestration proteins for tumor growth, and that trastuzumab is able to inhibit proliferation by down-regulating these p27 sequestering proteins.
It has also been proposed that decreased receptor signaling may be due to trastuzumab-mediated internalization and degradation of the receptor.28 However, it is unclear whether receptor down-regulation actually occurs, as other studies demonstrate that receptor levels are unchanged following treatment with trastuzumab.29,30 This observation is further supported by two studies indicating that trastuzumab does not increase the endocytosis of HER2, but instead, the antibody recycles to the cell surface with the internalized receptor.31,32
Another possible mechanism by which trastuzumab may induce apoptosis is via antibody-dependent cellular cytotoxicity (ADCC).33 The Fc domain of trastuzumab binds to the Fc gamma receptor on natural killer (NK) cells, resulting in NK-mediated cell lysis. In vitro studies demonstrate that NK cells are able to kill trastuzumabcoated HER2-overexpressing cells,34 and in vivo pilot studies reveal strong lymphoid infiltration in HER2-positive tumors treated preoperatively with trastuzumab.29 Since IL-2 leads to NK-cell expansion, it was hypothesized that administering IL-2 with trastuzumab might enhance ADCC. A small cohort of patients with metastatic HER2-positive breast cancer that had progressed on multiple prior systemic therapies were treated with low-dose IL-2 and trastuzumab, and while NK cell expansion and increased ADCC were seen, it did not correlate with clinical response.35
More recently, Musolino et al.36 provided additional evidence that trastuzumab-mediated ADCC is clinically important. Their group evaluated the effect of Fc gamma receptor polymorphisms on response to trastuzumab and taxane-based treatment in 54 patients with HER2-positive metastatic breast cancer. They found that the Fc gamma RIIIa-158 valine/valine (V/V) genotype, which more strongly elicits ADCC than other genotypes, was significantly correlated with response rate and progression free survival (PFS). This suggests that Fc polymorphisms may predict clinical outcome of patients with HER2-positive breast cancer receiving trastuzumab and further supports a role for ADCC in trastuzumab function.37 Prospective studies are needed to confirm these findings.
Many other mechanisms of action of trastuzumab have been proposed, including inhibition of angiogenesis,38 inhibition of ECD proteolysis,39 and inhibition of DNA repair.40 Further elucidating the mechanism by which trastuzumab mediates cytotoxicity will be important in improving our understanding of mechanisms of trastuzumab resistance and exploiting potential synergistic antitumor mechanisms.
Mechanisms of Trastuzumab Resistance
Based on the proposed mechanisms of action of trastuzumab discussed above, several theories have been put forward regarding the development of trastuzumab resistance, including loss of antibody binding, increased downstream signaling, and activation of alternative growth factor pathways.
Loss of Antibody Binding
Blockade of trastuzumab binding by MUC4, a cell surface mucin, has been implicated as a mechanism of resistance. Cancers often overexpress MUC4, and it has been hypothesized that this protects against antibody-mediated immune attack.41 MUC4 has also been
shown to bind to and potentiate phosphorylation of HER2.42 Breast cancer cells that overexpress MUC4 have a decreased level of interaction between HER2 and trastuzumab compared to cells that do not overexpress MUC4,43 and RNAi-mediated knockdown of MUC4 expression leads to increased binding of trastuzumab.44 Furthermore, treatment with anti-MUC4 antibodies reduces trastuzumab binding to HER2, suggesting that reduced antibody binding is due to steric hindrance from MUC4. In addition, MUC4 overexpression prevents HER2 from interacting with other proteins, such as EGFR or HER3, requiring these cells to find an alternative pathway to activate the PI3K and MAPK pathways.44 Therefore, it appears that MUC4 expression can lead to diminished trastuzumab binding and isolation of HER2 from its normal interaction partners.
shown to bind to and potentiate phosphorylation of HER2.42 Breast cancer cells that overexpress MUC4 have a decreased level of interaction between HER2 and trastuzumab compared to cells that do not overexpress MUC4,43 and RNAi-mediated knockdown of MUC4 expression leads to increased binding of trastuzumab.44 Furthermore, treatment with anti-MUC4 antibodies reduces trastuzumab binding to HER2, suggesting that reduced antibody binding is due to steric hindrance from MUC4. In addition, MUC4 overexpression prevents HER2 from interacting with other proteins, such as EGFR or HER3, requiring these cells to find an alternative pathway to activate the PI3K and MAPK pathways.44 Therefore, it appears that MUC4 expression can lead to diminished trastuzumab binding and isolation of HER2 from its normal interaction partners.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree