Hematopoietic Growth Factors
Jeffrey Crawford
Susan Blackwell
Hematopoietic growth factors are glycoproteins that stimulate the proliferation and development of clonogenic precursor cell populations. Through recombinant technology, hematopoietic growth factors administered at pharmacologic doses can provide significant clinical benefit for the cancer patient undergoing chemotherapy. Anemia can be reversed with the use of recombinant erythroid stimulating agents (ESAs) reducing transfusions and improving anemia-related symptoms such as fatigue. Neutropenia and its complications can be ameliorated by the use of myeloid growth factors (MGFs). These MGFs have also had a major role in the development of the technology of peripheral blood progenitor cell mobilization, reducing prolonged cytopenias after high-dose chemotherapy. Thrombopoietic agents have shown promising activity improving platelet counts in patients with immune thrombocytopenia (ITP), and studies in cancer chemotherapy patients are underway.
Much has been written and reviewed about the hematopoietic growth factors in terms of their biology and clinical application. This chapter summarizes the practicing oncologist areas in which hematopoietic growth factors have been demonstrated to be of clinical benefit, where they have not been shown to be beneficial, or potentially harmful, and where questions remain about the best use of these biologic agents in the supportive care of cancer patients.
ANEMIA AND ITS IMPORTANCE TO THE CANCER PATIENT
Chemotherapy administration results in anemia in most cancer patients, but anemia can also be related to various other factors, including a component of the anemia of chronic disease, and direct bone marrow infiltration, particularly in the case of hematologic malignancies and some solid tumors, as well as other secondary causes for anemia, such as blood loss, nutritional deficiencies, and hemolytic states, among others. The first category of chemotherapy-induced anemia (CIA) can be managed by transfusion support versus ESAs according to guidelines,1 whereas the latter group comprise anemia of cancer (AOC), where ESAs are not indicated. This heterogeneous group of disorders is best managed by the identification of and interventions directed at the underlying cause, when possible. For symptomatic patients with AOC without a reversible cause, transfusion is recommended rather than ESAs.1,2
ERYTHROPOIETIN
Erythropoietin is a lineage-specific distal-acting factor that stimulates maturation of committed erythroid progenitors to become mature erythrocytes.3 Carbohydrates, rich in sialic acid, make up 30% to 40% of the 34,000-Da molecule and are essential for its secretion, stability in the circulation, and biologic activity. Many earlier-acting factors, including stem cell factor/kit ligand, interleukin (IL)-11, IL-3, granulocyte colony-stimulating factor (G-CSF), and IL-6, all interact in the development of erythroid progenitor cells (burst-forming unit erythroid) from the quiescent stem cell. Granulocytemacrophage colony-stimulating factor (GM-CSF) and IL-3 are important factors in the differentiation of burst-forming unit erythroid to the more mature colony-forming unit erythroid. However, in this cascade of hematopoietic growth factors, erythropoietin is the critical hormone regulating final red cell production from the stage of the colony-forming unit erythroid to the basophilic erythroblast and ultimately to the erythrocyte.
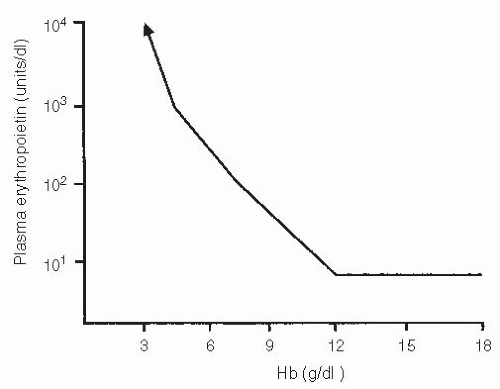
In humans, erythropoietin levels remain relatively constant until the hemoglobin level falls below 12 g per dl, at which time plasma erythropoietin levels increase4 (Fig. 6.1).
This observation suggests that rising erythropoietin levels may be a physiologic response to tissue hypoxia at hemoglobin
levels of <12 g per dl. Although this level is well above the level of anemia that is normally associated with cardiovascular symptoms, it is of interest that hemoglobin levels of 12 g per dl in cancer chemotherapy patients also represent a level associated with reduced fatigue and improved quality of life (QoL) in earlier clinical trials.5
levels of <12 g per dl. Although this level is well above the level of anemia that is normally associated with cardiovascular symptoms, it is of interest that hemoglobin levels of 12 g per dl in cancer chemotherapy patients also represent a level associated with reduced fatigue and improved quality of life (QoL) in earlier clinical trials.5
RANDOMIZED CLINICAL TRIALS OF EPOETIN ALFA
Recombinant human erythropoietin was purified and cloned in 1977 and then commercially produced as epoetin alfa.3 It was initially approved in 1987 as a treatment for anemia in chronic renal failure patients on dialysis and in 1990 as a treatment for patients with HIV-related anemia. In 1993, the indication was expanded to include chemotherapy-related anemia. The approval was based on a randomized placebo-controlled trial, with three subsets including anemia cancer patients who received platinum-based chemotherapy, nonplatinum-based chemotherapy, or no chemotherapy. The nonchemotherapy patients received a lower dose of epoetin alfa (100 units per kg) for a shorter period (8 weeks), and the hemoglobin improvement did not reach statistical significance. The chemotherapy groups received 150 units per kg epoetin alfa for 12 weeks, resulting in an improvement in hematocrit of approximately 6% points in cisplatin and in noncisplatin groups compared with placebo (P<.004). In addition, a statistically significant reduction was seen in transfusion requirements (P<.005) and overall QoL (P<.05) in the epoetin group compared with placebo. Adverse events were similar in the two groups and largely reflected the underlying disease and chemotherapy.
COMMUNITY-BASED TRIALS
To explore further the impact of anemia and its management on the patient undergoing cancer chemotherapy, three large open label community-based clinical trials were performed in patients with anemia in the setting of chemotherapy for either solid tumors or nonmyeloid hematologic malignancies.6,7,8 Although the lack of a control group limits some of the analyses, the sheer volume of patients, with >7,000 enrolled in the three trials, provides a very robust database to analyze the relationship of anemia to QoL and various other endpoints.
In the first two trials,6,9 epoetin alfa was administered at 10,000 units three times a week. As these trials were being conducted, preliminary experience suggested that a once-weekly dosage regimen might also be beneficial. Therefore, the third trial7 used a dose of epoetin alfa of 40,000 units subcutaneously once a week. The endpoints of all three studies included the effect on hemoglobin, transfusion requirements, and QoL as measured by the linear analogue scale. The latter two trials also included more detailed analysis of QoL using the Fact-AN.
Despite some of these methodologic differences, the results of these three trials are remarkably similar. In all three studies, the median hemoglobin at the time of enrollment was 9.2 to 9.5 g per dl. Over the course of 4 months of therapy, the mean improvement from baseline was 1.8 to 2.0 g per dl (P<.001). The percent of patients requiring transfusion was reduced from 16%-21% to 5%-10% in the three trials. Approximately 65% of patients achieved a 2.0 g per dl or greater improvement in their hemoglobin levels. The weekly administration strategy appeared to be as effective as three times a week and has become the clinical standard for administration. All three studies showed an approximately 30% or greater improvement in the patient’s self-reported measures of energy, ability to perform daily activities, and overall QoL. These improvements were directly correlated with improvements in hemoglobin, regardless of whether patient had a complete response, partial response or stable disease from chemotherapy. In patients with progressive disease, QoL improvement was blunted despite hemoglobin response.
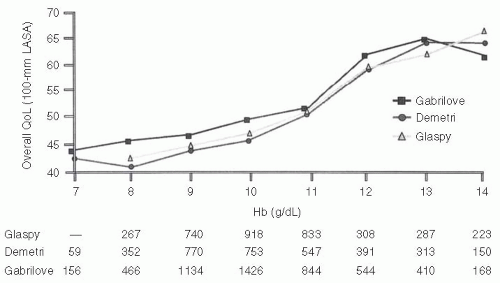
Figure 6.2 outlines the relationship between hemoglobin and overall QoL in the cross-sectional analyses performed in these three studies.5 This represents a compilation of measures of QoL by the linear analogue scale at the time of hemoglobin measurement across the course of the study. Although these data are unblinded, hemoglobin level appears to have a very consistent effect on QoL measures across all three studies. Below a hemoglobin of 8 to 10 g per dl, the “physiologic” levels at which transfusions are often considered, little improvement in the QoL occurs. However, in the range of 10 to 12 g per dl, there seems to be a more direct relationship, with higher levels of QoL associated with higher levels of hemoglobin. Above a hemoglobin of 12 g per dl, there is little further improvement in the QoL. This incremental analysis helped to define a hemoglobin of 12 g per dl as an optimal level in earlier studies from a QoL standpoint.
Furthermore, another randomized placebo-controlled phase III trial in CIA patients in Europe validated these benefits in QOL associated with hemoglobin improvement for patients receiving epoetin, compared with placebo patients who demonstrated a decline in QOL during chemotherapy.8
DARBEPOETIN ALFA
Darbepoetin alfa is a 165 amino acid protein that stimulates erythropoiesis but differs from erythropoietin and contains 5-N-linked oligofaccharide chains, whereas recombinant human erythropoietin contains three chains. These additional carbohydrate chains increase the molecular weight of darbepoetin alfa to 37,100 Da, resulting in a longer half-life, decreased receptor affinity, and increased biologic activity. In both animal and human models, darbepoetin alfa was shown to exhibit approximately three times longer half-life than that of recombinant human erythropoietin when given intravenously. Darbepoetin alfa has been approved for the treatment of anemia associated with chronic renal failure including patients on dialysis and patients not on dialysis, as well as for the treatment of anemia in patients with nonmyeloid malignancies and anemias due to administration of chemotherapeutic agents. Adverse effects associated with recombinant human erythropoietin and
darbepoetin alfa are similar when comparing package insert information.10
darbepoetin alfa are similar when comparing package insert information.10
Randomized placebo-controlled double blinded multinational studies confirm the safety and efficacy of darbepoetin alfa in reducing requirement for RBC transfusions in patients undergoing chemotherapy.11 Additional studies have shown that darbepoetin alfa could be given every 2 weeks effectively at doses of 3.5 and 5.0 mg per kg with a 2 g per dl rise in hemoglobin achieved in most patients.12 Ultimately, a fixed dose of 200 mcg every 2 weeks or 500 mcg every 3 weeks was approved, which allowed for synchronizing of many chemotherapy treatments and a more convenient alternative for patients.13,14
ANEMIA, SURVIVAL AND ERYTHROPOIETIC STIMULATING AGENTS
Anemia is a very common occurrence in patients with cancer and cancer patients receiving chemotherapy. AOC is associated with decreased survival in many malignancies, including lung cancer, head and neck cancer, prostate cancer, cervical cancer, lymphoma and multiple myeloma, and needs to be considered as distinct entities as AOC and CIA. From a review of over 200 papers addressing this subject, the overall estimate of risk of death was increased by 19% in anemic patients with lung cancer, 75% in anemic patients with head and neck carcinoma, 67% in anemic patients with lymphoma, and 47% in anemic patients with prostate cancer.15
From these studies, the association of anemia with poor survival in cancer patients was well established but not the causality. Anemia may be a marker of disease extent, other comorbid disease, poor performance status, and so on, which may be the reason for poor survival. On the other hand, anemia may result in altered tumor blood flow, increased tumor hypoxia, and other factors that may lead to a more malignant phenotype or poorer response to treatment such as radiation or chemotherapy.
With the ability of ESAs to restore hemoglobin to normal levels, reduce transfusion requirements, and improve signs and symptoms of anemia and QoL in the CIA patient, there was substantial interest in seeing if correction of hemoglobin levels would in fact also lead to improved survival. Most of the randomized trials of ESAs in the AOC did not include survival as an endpoint and often involved heterogeneous populations of patients and treatment regimens making any conclusions about survival outcome difficult. Therefore, most “survival” trials focused not on a population of patients with AOC or chemotherapy-related anemia, but in fact in patients with minimal or no anemia to see if use of ESAs to higher target hemoglobin levels would result in improved outcomes.
However, the result of these investigations had the opposite outcome in several studies, including decreased survival and increased mortality with the use of ESAs.
The Cochrane meta-analysis of ESAs initially showed no definite impact on survival, positively or negatively,16,17 until the more recent studies were included.18 Very few of the initial trials in the meta-analyses were designed with survival as a primary endpoint. The two trials that initially raised concern and led to an initial Oncology Drug Advisory Committee (ODAC) meeting in 2004 were the BEST trial of epoetin alfa in women with metastatic breast cancer19 and the ENHANCE trial of epoetin beta in patients with head and neck cancer receiving radiotherapy.20 Both of these trials treated patients to above standard hemoglobin
levels and both were associated with worsening survival in the ESA-treated group. A review for the FDA by the ODAC of the totality of data for all the ESAs reconfirmed that the risk of thrombovascular events was increased with the relative risk of 1.5. At the time, the recommendation was that ESAs should be used within the labeled indication of correction of anemia with a target of 12 g per dl and that further survival studies were indicated.
levels and both were associated with worsening survival in the ESA-treated group. A review for the FDA by the ODAC of the totality of data for all the ESAs reconfirmed that the risk of thrombovascular events was increased with the relative risk of 1.5. At the time, the recommendation was that ESAs should be used within the labeled indication of correction of anemia with a target of 12 g per dl and that further survival studies were indicated.
However, additional trials raised concerns and led to a second ODAC meeting of May 10, 2007. The Danish Head and Neck Cancer radiation trial (DAHANCA)21 was similarly designed to the ENHANCE trial, evaluating high hemoglobin in radiation patients. This trial was stopped when an interim analysis demonstrated futility for benefit and a detrimental impact on tumor outcomes in the ESA-treated group.
Another trial was a randomized, double blind placebo-controlled trial of epoetin alfa in patients with non small cell lung cancer (NSCLC), who had anemia either related to the cancer (AOC) or to chemotherapy (CIA), but most of the patients were in the AOC category.22 The goal was to restore hemoglobin to the 12 to 14 g per dl range. The data safety and monitoring board stopped the trial early to look for an increased rate of venous thromboembolic (VTE) risks. A reduced survival was seen in the ESA-treated patients.
The trial that brought the most attention to ODAC was a large prospective, placebo-controlled darbepoetin trial in patients with AOC.23 In this trial, closed by the sponsor at an interim analysis, a shorter survival was seen in the treatment group. The patients receiving darbepoetin had an escalated risk of VTE, consistent with other trials, and patients with VTE did not appear to account for the differences in survival. Furthermore, analysis by disease and other patient characteristics did not clearly identify the group at risk.
At ODAC, these current trials as well as past trials were reviewed. Transfusion avoidance was recognized as a patient benefit. QoL benefits of ESAs, although recognized in the European community, have not been a labeled indication in the United States for the use of these agents, and the methodology to perform and interpret their results lacks consensus. In addition to these trials raising safety issues of ESAs in breast cancer, head and neck cancer, and lung cancer, additional studies in lymphoproliferative disorders24 and cervical cancer1 also demonstrated adverse impacts on treatment outcomes.
The updated Cochrane meta-analysis including these studies did demonstrate a negative impact on survival, along with an increased risk of deep venous thrombosis.18 Based on these expanded data, the FDA revised the label of the ESAs and, in conjunction with the sponsors, has developed a REMS program (risk evaluation and mitigation strategy) that limits the use of these agents to patients receiving chemotherapy in the noncurative setting, with hemoglobin levels <10 g per dl, with adequate consent process to discuss the risks and benefits of ESAs versus transfusions. This has resulted in a marked decline in the use of ESAs in oncology patients in the United States.
The ESA story highlights the importance of fully understanding the biology of these agents. Whether tumors have functional erythropoietin receptors is controversial. Studies that look at tumor blood flow and oxygenation have suggested a potential explanation for a negative impact of high hemoglobin levels through increased viscosity, but these are still preliminary. Clinically, more studies are needed to help predict patients at risk for deep venous thrombosis as well as to develop preventive strategies. Within the labeled indication for correction of anemia, QoL data acceptable to regulatory agencies are needed to demonstrate this benefit. Meanwhile, the reader should follow treatment guidelines in the management of these patients and look for updated recommendations, which have occurred frequently in this setting.1,2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree