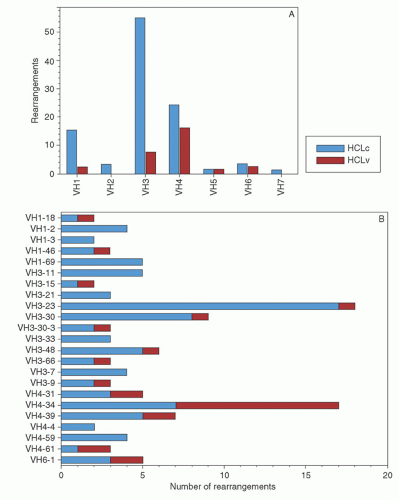
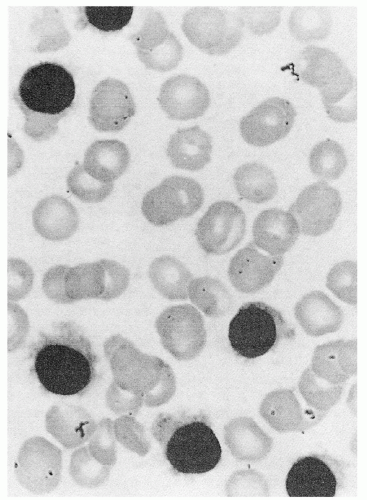
appears to be the most effective way of inducing hairy cell proliferation.47 This technique allowed cytogenetic analysis in 42 of 43 cases and demonstrated that clonal abnormalities were present in 19% of cases and involved numeric or structural abnormalities in chromosomes 5, 7, and 14. In contrast, abnormalities of chromosome 5 were not observed in the HCL variant, and translocations more frequently involved either chromosome 2 or 14.48
Syndecan3, Cyclin D1, IGFBP, and NUDT6.24 The proposed effects of these changes on the pathophysiology of HCL are outlined in Table 91.1. More recently, a heterozygous mutation of GTG to GAG at codon 600 of the BRAF gene has been observed in typical HCL producing a V600E variant protein, in which glutamate substitutes for valine at position 600.58, 59, 60, 61, 62 BRAF is a serine-threonine kinase and the variant protein (V600E) demonstrates increased activity resulting in enhanced phosphorylation and activation of mitogen-activated protein-ERK kinase (MEK) and extracellular signal-regulated kinase (ERK), which increase cell survival and proliferation by increasing the expression cyclin D1 and decreasing p27.58 The functionality of the variant protein was demonstrated in primary hairy cells by showing the presence of phosphorylated MEK and ERK in these cells, and demonstrating that the phosphorylation was decreased using the BRAF inhibitor, PLX-4720.58 Initial studies suggested that all patients with classical HCL contained a V600E; however, a more recent study demonstrated that 82% of patients with classical HCL express BRAF, whereas the V4-34 subtype and HCL variant do not express BRAF.21 Thus, BRAF inhibitors may not be useful in the treatment of the aggressive V4-34 HCL subtype of the HCL variant.
TABLE 91.1 MECHANISMS FOR HAIRY CELL LEUKEMIA FEATURES | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
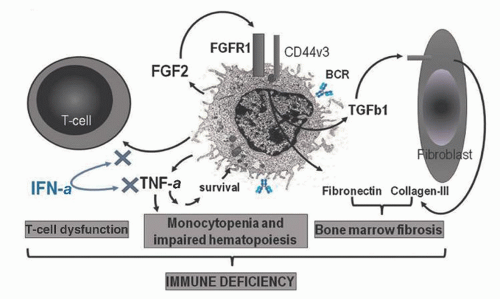
quantities in the plasma and marrow of patients with HCL, and this also contributes to the marrow fibrosis by inducing the production of collagen and reticulin by adjacent fibroblasts.82
TABLE 91.2 HAIRY CELL LEUKEMIA: CLINICAL MANIFESTATIONS | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
with HCL.83, 111, 112, 113, 114, 115 In one series of patients, these complications were second only to infection as a cause of morbidity.83, 112 The onset may occur any time during the course of the disease and is not related to the tumor burden. Most frequently, patients present with arthritis, arthralgias, palpable purpura, or nodular skin lesions resulting from cutaneous vasculitis, and low-grade fever.83, 112, 114 Occasionally, patients may have involvement of the lung, liver, intestine, and kidney, with a clinical picture that resembles polyarteritis nodosa.83, 112, 113, 115 These patients often have fever, malaise, and weight loss, and a co-existent infection must be ruled out.83, 112 If skin lesions are present, the diagnosis can be confirmed by biopsy, which usually shows changes compatible with a diagnosis of polyarteritis nodosum or leukocytoclastic vasculitis; occasionally, a vasculitis related to the invasion of the vessel wall by hairy cells occurs; this may appear very similar to polyarteritis nodosa with the presence of aneurysms.114 In some organs, such as the lung, a granulomatous vasculitis may be found.112 Angiography may reveal peripheral aneurysms.112 Antinuclear antibodies, rheumatoid factor, immune complexes, and hepatitis B antigen are variably positive.111 Cryoglobulinemia has been detected in some patients.116, 117 It has been postulated that the increased incidence of vasculitis in HCL may be related to infections with hepatitis B and other viruses, cross-reactivity of antibodies against hairy cells with epitopes on endothelial cells, and decreased clearance of immune complexes by the impaired immune system.114
TABLE 91.3 HAIRY CELL LEUKEMIA: UNUSUAL CLINICAL MANIFESTATIONS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 91.4 HAIRY CELL LEUKEMIA: LABORATORY MANIFESTATIONS | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
B-ly 7, the antibodies HML-1, Ber-ACT8, and LF61 can also recognize CD103. Two further antibodies, RAB-I and anti-HC2, are also relatively specific for hairy cells.6 CD123 (α chain of the IL-3 receptor) is present in 95% of cases with classical HCL and is only rarely detected in other chronic lymphoid malignancies.142, 143, 144 Hairy cells are also typically strongly FMC7 positive (an epitope of CD20), negative for CD79b (an epitope of B-cell receptor β chain), and CD27, CD10, and CD38 are rarely positive.142 In summary, the diagnostic immunophenotypic features for HCL are monoclonal B-cells that are positive for CD11c, CD25, CD103, and CD123.142
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree