Chapter Outline
Introduction: types of laboratories 547
Organisation of clinical laboratory services 547
Level 1: primary and subdistrict laboratories 548
Level 2: intermediate (district) level laboratories 548
Level 3: regional and provincial hospitals 548
Level 4: national referral and teaching hospitals 548
Availability of haematology tests at each level 548
Microscopes 549
Care of the microscope 549
Essential haematology tests 549
Maintaining quality and reliability of tests 550
Quality control of a test method (technical quality) 550
Internal quality control 550
External quality assessment 551
Basic haematology tests 551
Haemoglobinometry 551
Direct reading haemoglobinometers 551
Haemoglobin colour scale 551
Packed cell volume 552
Manual cell counts using counting chambers 552
Total white blood cell count 552
Platelet count 554
Errors in manual cell counts 555
Standardised counting chambers 555
Accurate dilutions 555
Microscopy artefacts 555
Peripheral blood morphology 555
Modified (one-tube) osmotic fragility test 556
Haemoglobin E screening test 556
Sickle cell screening test 556
Tests for paroxysmal nocturnal haemoglobinuria 556
Reagents for prothrombin time and activated partial thromboplastin time 556
Laboratory support for management of HIV/AIDS: CD4-positive T-cell counts 557
Laboratory management 557
Interlaboratory communication 557
Specimen transport 557
Point-of-care tests 557
Staff training 557
Clinical staff interaction 558
Facility management teams 558
Health and safety 558
Introduction: types of laboratories
In most countries, there are likely to be some laboratories with limited resources, but in under-resourced countries, the majority of laboratories face chronic shortages of trained staff, low morale, inadequate and poorly maintained equipment and erratic supplies of reagents and essential supplies. These factors have a major impact on the range and quality of services offered. Many laboratories lack the highly sophisticated equipment found in better resourced settings and some still operate using predominantly manual techniques. The most peripheral laboratories tend to be multifunctional, with staff performing a range of haematology, parasitology, clinical chemistry and microbiology tests. A blood transfusion service is usually available at larger facilities and, although nationally organised blood services are expanding, much blood for donation is still sourced and used at individual hospital level. In practice this means that laboratory staff may be responsible for donor selection, blood collection and issuing of blood. If there is no separate system of public health laboratories, clinical laboratories may also be required to provide reliable health surveillance data for epidemiological and public health monitoring and to investigate disease outbreaks and refer samples for confirmation.
In under-resourced countries, these difficulties are compounded by the high burden of infectious diseases such as malaria, human immunodeficiency virus infection/acquired immune deficiency syndrome (HIV/AIDS), tuberculosis and neglected tropical diseases such as schistosomiasis and helminthiasis. There are also additional pressures from external agencies such as donors, funders and research projects which may not align closely with local public health priorities. Parasitological diagnosis is now recommended for all suspected cases of malaria to prevent overdiagnosis and reduce inappropriate use of antimalarial drugs. World Health Organisation (WHO) guidelines state that malaria treatment based on clinical grounds should only be given if diagnostic testing is not immediately accessible within 2 h of patients presenting for treatment. In higher level or specialised treatment centres, the diagnosis of leishmaniasis may require aspiration of bone marrow or splenic pulp. The diagnosis of tuberculosis may require aspiration and culture of bone marrow and trephine biopsy examination, especially in patients who are HIV positive, in whom sputum tests for acid-fast organisms are frequently negative. The decision to initiate antiretroviral therapy, to switch to second-line drugs and the monitoring of therapy require regular haemoglobin concentration (Hb) measurements, CD4-positive lymphocyte counts (or percentages for paediatric care) and, ideally, plasma viral load determinations (HIV ribonucleic acid (RNA) monitoring), although provision of some of these tests is challenging for low-income countries. ,
The purpose of this chapter is to provide guidance for an effective haematology service at the different levels of the healthcare system in resource-limited countries. In planning such services, it is necessary to determine what tests are needed at each level, to ensure that tests are reliable and accurate and to establish effective referral networks for patients and samples. In some cases this may involve regional and international partnerships if resources for quality diagnoses are not available in-country.
Organisation of clinical laboratory services
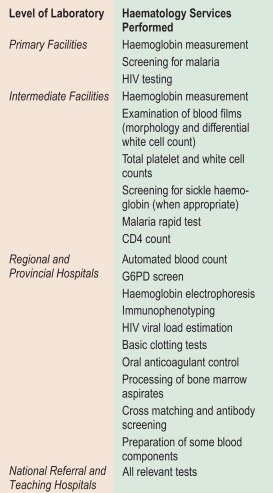
In under-resourced countries clinical laboratory services may be considered at four levels according to size, staffing and services offered. These levels are: primary facilities, including subdistrict hospitals and health centres (Level 1); intermediate facilities, including district and county hospitals (Level 2); regional and provincial hospitals (Level 3); and national referral and teaching hospitals (Level 4) ( Fig. 26-1 ).
Level 1: primary and subdistrict laboratories
Level 1 laboratories provide first-line diagnostic services to support patient management decisions, initial public health investigations and specimen referral. Laboratory staff may comprise one or two qualified laboratory technicians supported by assistants who often have little or no formal training but have learnt various techniques at the bench. In very peripheral settings, there may be no formal laboratory, and some point-of-care tests are carried out by nurses or assistants with limited training. There is increasing availability of point-of-care diagnostics for an expanding repertoire of conditions, which while enabling more patients to access diagnostic tests, requires adherence to standards to ensure test quality, satisfactory operator performance and correct use of results for patient treatment. This ‘task shifting’ may be an increased burden for already overworked staff and requires intensive supervision and quality monitoring. Maintenance of equipment in rural areas is often difficult, which can significantly compromise the quality of results or interrupt services altogether. Haematology laboratory tests at this level generally include a method for measuring Hb; microscopy for malaria and other blood parasites, blood cell morphology; and HIV and sickle cell screening.
Level 2: intermediate (district) level laboratories
Intermediate level laboratories are usually multipurpose, and perform microbiological and biochemical as well as haematological tests. Laboratory staff may comprise a limited number of qualified laboratory technicians or biomedical scientists. Equipment available for haematology may include a microscope, centrifuge and basic colorimeter for measurement of Hb. In some intermediate laboratories more sophisticated equipment such as automated haematology and clinical chemistry analysers are available, but long-term sustainability requires funding for regular servicing and maintenance at the time of procurement and installation and purchase of appropriate reagents. In the absence of access to a national blood service, intermediate hospital laboratories are responsible for blood transfusion services with the laboratory expected to perform blood grouping and crossmatching as well as screening for HIV, hepatitis B virus (HBV), hepatitis C virus (HCV) and syphilis.
Level 3: regional and provincial hospitals
At this level, laboratory staff have usually received multidisciplinary training and specialist laboratory staff are available. Level 3 laboratories are usually multipurpose, performing microbiological, biochemical and haematological tests, as well as some more specialised tests. Automated haematology and chemistry equipment, and tests such as CD4-positive lymphocyte counting and coagulation tests are available.
Level 4: national referral and teaching hospitals
At this level, laboratory staff have usually received multidisciplinary training and some of the senior staff have received postgraduate training in a specialist laboratory discipline. Each laboratory usually has a specialist technical head who works closely with the clinician responsible for laboratory services. Equipment generally available includes centrifuges, colorimeters, microscopes, haemoglobin electrophoresis equipment, automated haematology and clinical chemistry and blood grouping and crossmatching analysers, and possibly high performance liquid chromatography and blood bank centrifuges for the separation of blood components.
Availability of haematology tests at each level
In under-resourced countries, haematology tests available at the different levels of healthcare vary widely and depend on local clinical and public health needs as well as availability of equipment and qualified technical laboratory personnel. The following is a general description of the haematology-related tests that are likely to be required at each level.
Level 1
- •
Hb measurement by a manual method (see p. 22)
- •
Malaria and other blood parasite testing on thick and thin peripheral blood films (see p. 101) or rapid malaria diagnostic test (see p. 102)
- •
Peripheral blood cell morphology, especially to identify the cause of anaemia
- •
Sickle cell screening
- •
Testing for HIV for blood transfusion screening
Level 2
- •
Hb measurement (see p. 19)
- •
Peripheral blood cell morphology
- •
Total white blood cell counts (see p. 36)
- •
Differential white cell count (see p. 25)
- •
Platelet estimates (usually from blood film)
- •
CD4-positive lymphocyte count (see p. 557)
- •
Malaria and other blood parasite testing by thick and thin peripheral blood films (see p. 101) or rapid diagnostic test for Plasmodium falciparum and other species (see p. 102)
- •
Screening test for sickle haemoglobin in areas where this is relevant (see p. 297)
- •
Blood grouping and compatibility testing ( Chapter 21 )
- •
Testing for HIV, HBV, HCV and syphilis infection for blood transfusion screening
- •
Some larger laboratories may be able to provide automated measurements of Hb, mean cell volume (MCV), mean cell haemoglobin (MCH), mean cell haemoglobin concentration (MCHC), white blood cell total count (WBC), differential count and platelet count (see p. 30).
Levels 3 and 4
In addition to tests carried out at level 2, the haematology services offered by level 3 and 4 laboratories may include the following:
- •
Automated Hb, MCV, MCH, MCHC, WBC and differential counts, platelet counts (see p. 30)
- •
Haemoglobin electrophoresis or high performance liquid chromatography ( Chapter 14 )
- •
Haemoglobin A 2 and haemoglobin F measurements (see pp. 302 and 306)
- •
Glucose-6-phosphate dehydrogenase screen (by fluorescent spot or methaemoglobin reduction method) or molecular methods (see p. 238)
- •
Flow cytometric immunophenotyping (see Chapter 16 )
- •
Polymerase chain reaction (PCR) or other method for diagnosis of mutations associated with haematological malignancies (see Chapter 23 )
- •
HIV plasma viral load estimations
- •
Staining of bone marrow films for morphological assessment (see p. 52) and estimation of iron status (see p. 120)
- •
Bone marrow trephine biopsy examination (see p. 120)
- •
Identification of blood group antibodies (see Chapter 21 )
- •
Basic clotting screen (prothrombin time (PT), thrombin time (TT) and activated partial thromboplastin time (APTT)) and possibly thrombophilia tests (see p. 410)
- •
Oral anticoagulant control (see p. 426)
- •
Separation of whole blood into packed cells, plasma and, occasionally, platelets and cryoprecipitate.
Microscopes
The microscope is the most important piece of laboratory equipment in under-resourced countries and is essential for the diagnosis of anaemia, malaria and other blood parasitic infections and for performing differential white blood cell counts and sickle cell screening tests. Reliable assessment of morphological features requires a microscope that is clean and correctly set up with aligned lenses and an electric light source (either inbuilt or reflected light) to ensure clear images, especially at high magnification. Failure to maintain microscopes to a high standard through routine user maintenance or, ideally, with regular professional servicing, can lead to inaccurate diagnoses and inefficient use of technician time. , Routine maintenance of the microscope is described on p. 46.
Care of the microscope
In hot, humid climates, if no precautions are taken, fungus may grow on the surface of the lenses, in the grooves of the screws and under the paint. This can be prevented by placing the microscope every evening in an airtight dust cover together with silica gel. Dry the silica as necessary and reuse it. An alternative method is to place the microscope in a warm cupboard with a tight-fitting door, heated by a 40-watt light bulb. Check that the temperature inside the cupboard is at least 5 °C warmer than that of the laboratory, but take care that it does not overheat.
In hot, dry climates, the main problem is dust. Fine particles work their way into the threads of the screws and under the lenses. This can be avoided as follows:
- 1.
Always keep the microscope under a dustproof plastic cover when not in use.
- 2.
At the end of the day’s work, clean the microscope thoroughly by blowing air onto the lenses and moving parts.
- 3.
Finish cleaning the lenses with a lens brush or fine paintbrush. If dust particles remain on the surface of the objectives, remove with a clean lens tissue.
Essential haematology tests
Despite the relatively high cost of running a laboratory service and the low per capita healthcare budget in under-resourced countries, there is little guidance available on how to make rational decisions on the choice of ‘essential’ laboratory tests. , Decisions about which tests to provide must be made in consultation with laboratory professionals and clinical staff. The selection of ‘essential’ tests at each level should be based on the clinical and public health needs of the local community and the availability of qualified clinical and laboratory staff, as well as the availability of funds. Essential test packages are usually defined as part of national policy and standards, taking into consideration medium- and long-term trends and the requirements of disease control programmes.
To ensure cost-effectiveness of the laboratory service, tests with no proven value should be eliminated and new tests for which there is independent evidence of clinical usefulness should be introduced, as described in Chapter 24 . Tests that provide objective qualitative or quantitative information are preferred. Although it is not possible to draw up a list of essential tests applicable to all countries, or even to different regions within a country, the following aspects should be considered.
Cost per test
Often the cost of a test is calculated from the price of reagents divided by the number of tests performed. However, this oversimplifies the situation and is not accurate enough to form the basis for national policy decisions and budget allocation. The factors that need to be taken into account when calculating the total annual costs for a laboratory are given in Chapter 24 .
Diagnostic reliability
It is important to know the sensitivity and specificity of a test and its predictive values (as calculated on p. 568) when selecting a laboratory test for clinical use. This information may be provided by manufacturers but may not be locally applicable, and local test evaluation data are usually not available in under-resourced countries. In some countries, the ‘gold standard’ diagnostic services needed to determine ‘true positive’ and ‘true negative’ data in the local context are also lacking.
The quality of all tests carried out by a laboratory should be regularly monitored. Systems for achieving this are well established (see Chapter 24 ) but are not easily implemented in under-resourced settings. The quality of a test influences its usefulness as well as its utilisation by clinicians and community members. For example, if the result of a test in routine practice is correct only 80% of the time, then one in five tests will be wasted, reducing the effectiveness of the test by 20%. Furthermore, an inaccurate test may result in a patient receiving inappropriate treatment. Clinicians and the general public are increasingly aware of the need for reliable services. Clinicians may not order tests or use the results in patient management decisions if they do not trust the quality of the laboratory results, and poor quality healthcare may deter patients from using health facilities.
Clinical usefulness
An assessment of the clinical usefulness of a test should be carried out by an independent clinician who is familiar with local diseases and the diagnostic support services available. This assessor needs to compare actual clinical practice with locally agreed ‘best practice’ or, if available, local guidelines. From observation of a range of clinical interactions, the percentage of times that ideal practice is followed can be calculated. For example, transfusion guidelines may recommend that, apart from specified clinical conditions, transfusions should be given only to children with an Hb of < 50 g/l. The assessor can record how many children with Hb below this level failed to receive a transfusion and how many transfusions were given without waiting for the Hb result or at an inappropriate Hb level. For each test, the assessor needs to judge whether the test has been appropriately requested and has been used to influence patient management or public health decisions. The percentage of tests that are not used to guide clinical decisions will provide a figure for ‘clinical wastage’ of the test that can be entered into a simple formula ( Chapter 24 ).
Maintaining quality and reliability of tests
Paradoxically, it is in under-resourced laboratories, where equipment and supplies are limited and training and supervision may be minimal, that the level of skills and motivation required to maintain a good-quality service need to be highest. Even the most basic of laboratories should ensure that procedures are in place to monitor quality (see Chapter 25 ). In addition to monitoring the technical quality of each test, quality management and improvement processes should be in place throughout the laboratory. The Centers for Disease Control and Prevention/World Health Organisation (CDC/WHO) WHO Guide for the Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA) in the African Region , being rolled out in developing countries provides a structured quality improvement process comprising workshop-based teaching, laboratory quality improvement projects, on-site mentoring and regular assessments over a period of time (up to 2 years). Standard operating procedures (SOPs) (see p. 523) should be available for every procedure performed in the laboratory; these can be adapted from existing SOP ‘models’. In addition to providing standardised techniques, SOPs are excellent teaching resources, and adherence to these procedures will minimise errors. SOPs need to be regularly reviewed and updated to keep pace with technical developments and changes in local circumstances (e.g. changes in availability of reagents or equipment).
Quality control of a test method (technical quality)
Methods for the control of various haematology tests are described in Chapter 25 but some may need to be adapted to specific local circumstances in resource-poor settings. For example, if commercial controls for sickle cell tests are not affordable, each batch of tests should include known positive and negative samples from a previous batch of tests; for monitoring constancy of Hb measurements, a high and a low value sample can be retested several times during the day.
Internal quality control
Internal quality control is a system within an individual laboratory for ensuring that the technical elements of the test are of acceptable quality. Monitoring of quality by processing control samples and plotting a control chart (see p. 539) will highlight problems within the system which need to be investigated and corrected. For example, an inaccurate differential white cell count may be due to problems with sample collection and handling, slide preparation, fixing and staining, morphological interpretation and microscope quality as well as inadequate microscopy technique. Measures such as the introduction of SOPs, in-service training and equipment maintenance schedules can help to improve performance and reduce inaccuracies.
External quality assessment
The principles of external quality assessment (EQA) are described on p. 539. Since EQA schemes depend on effective distribution of samples and timely return of results, poor communications and transport facilities may make it logistically difficult to conduct EQA for under-resourced laboratories. Although participation in international quality assessment schemes is beyond the resources of most peripheral laboratories, national authorities need to ensure participation of laboratories at all levels in national schemes, even if this initially requires external support. EQA schemes should be educational and supportive and used to identify poorly performing laboratories that require assistance, as well as to detect inaccuracies in test methods or performance, as part of postmarketing surveillance. Laboratories may also exchange samples and results with neighbouring facilities as part of EQA. Laboratories should also take advantage of supervisory visits, for example by tuberculosis or malaria programme officers, to review EQA or difficult slides, and exchange samples and results with other laboratories. Accreditation schemes (see p. 540), either national or local, can be set up to formally recognise laboratories that are performing well and to assist those that are not. Stepwise – rather than pass/fail – accreditation schemes provide a better measure of performance and motivate laboratories to improve their services.
Basic haematology tests
Haemoglobinometry
Various methods for estimating Hb are given in Chapter 3 . All methods, except colour comparison methods, require a power source, either mains electricity, batteries charged by solar or generator power or battery cells. The most accurate method available in under-resourced laboratories is usually the haemiglobincyanide (formerly cyanmethaemoglobin) method performed in a manual colorimeter. However, this requires technical expertise to carry out accurate pipetting and dilutions and to prepare standard calibration curves. Use of preset haemoglobinometers removes the need for calibration curves, but machines may still need to be set correctly and must be quality assured.
Haemoglobin testing kits for home use are becoming increasingly available but are of variable quality and user-friendliness. Methods for measuring Hb that are robust, accurate and are used by health workers in peripheral settings are described below.
Direct reading haemoglobinometers
HemoCue blood haemoglobin system
HemoCue Blood Haemoglobin System (see Chapter 3 ) is a battery- or mains-operated portable, direct read-out machine that uses disposable dry chemistry cuvettes. Measurements are precise and accurate but only if specified cuvettes are used, the reading surfaces are kept clean and the cuvettes are properly filled with blood. Unlike most other systems, measurement does not require predilution of the sample. Although the use of unique disposable cuvettes makes this method relatively expensive, the cuvettes for the new Hb 301 version are generally cheaper than those for the Hb 201 and are designed for adverse climatic conditions. HemoCue is fast and simple to use so some costs may be offset by savings on training and supervision time. Disadvantages also include need for an effective supply chain to ensure availability of cuvettes and waste management of used cuvettes. In addition, Hb 310 is not suitable for EQA using haemoglobin lysate.
Haemoglobin colour scale *
* Available from Teaching Aids at Low Cost; www.talcuk.org/accessories/haemoglobin-colour-scale.htm (accessed April 2015).
Many colour comparison methods have been available in the past, but these have become obsolete because the colours were not sufficiently comparable with blood or were not durable. The haemoglobin colour scale was developed for anaemia screening where there is no laboratory. It consists of a set of printed colour shades on a chart representing Hbs between 40 and 140 g/l. The colour of a drop of blood collected onto a specific type of absorbent paper is compared with the colours on the chart ( Fig. 26-2 ). It is intended for detecting the presence of anaemia and estimating its severity in 20 g/l increments. The scale has been tested in resource-poor settings but appeared to be less accurate at mild anaemia levels, where clinical assessment is also less sensitive, and more studies are needed to demonstrate whether it is superior to clinical diagnosis. The instructions should be followed carefully because poor lighting, poor eyesight, allowing the blood spot to dry out and using the incorrect type of absorbent paper for the test strips can have detrimental effects on the results.

Other colour comparator methods still in use include the Sahli method where the colour of blood after mixing with a chemical is compared against a range of standard colours. The same problems associated with inaccuracy and subjectivity apply to these methods, and their use is not recommended.
Packed cell volume
Packed cell volume (PCV) can be used as a screening test for anaemia; however it is not a substitute for a well-performed Hb measurement. In addition to technical problems (outlined on p. 23) there are specific problems with this method in resource-poor settings that may lead to errors in estimating the PCV. Poor-quality sealant can result in the tubes leaking during centrifugation. Because the microhaematocrit tubes are difficult to label, samples may get mixed up in the centrifuge, especially when pressure of work is high and supervision is poor. Erratic power supplies, lack of devices for measuring g forces and poor equipment maintenance may result in inadequate centrifugation with incomplete packing of the red cells. In addition, tubes are disposable and require good supply management and waste disposal systems.
Manual cell counts using counting chambers
Visual counting of blood cells is an acceptable alternative to electronic counting for white blood cells but it is not recommended for red blood cell counts because the number of cells that can be counted within a reasonable time in the routine laboratory (e.g. about 400) will be too few to ensure a sufficiently precise result (see below). Although it is possible to perform a manual platelet count, in practice, sufficient information about the platelet count may be obtained from microscopic examination of the peripheral blood film.
Counting chambers
The visibility of the rulings in the counting chamber is as important as the accuracy of calibration, so that chambers with a ‘metallised’ surface and Neubauer or Improved Neubauer rulings are recommended. These have nine 1 × 1 mm ruled areas, which, when covered correctly with the special thick coverglass, each contain a volume of 0.1 μl of diluted blood ( Figs 26-3 and 26-4 ). Coverslips designed for mounting of microscopy preparations must not be used with counting chambers as they may bow, reducing the volume of the sample. The sample is introduced between the chamber and the coverglass using a pipette or capillary tube, and the preparation is viewed using × 40 objective and × 10 eyepieces. With Neubauer and Improved Neubauer chambers, the cells in 4 or 8 horizontal rectangles of 1 × 0.05 mm (80 or 160 small squares) or in 5 or 10 groups of 16 small squares are counted, including the cells that touch the bottom and left-hand margins of the small squares.
Total white blood cell count
To make the counting of white cells easier, diluted whole blood is mixed with a fluid to lyse the red cells and stain the white cell nuclei deep violet-black.
Method
Make a 1 in 20 dilution of blood by adding 0.1 ml of well-mixed blood (lack of adequate mixing is a major source of error) to 1.9 ml of diluent †
† Or a proportionately smaller volume: 0.02 ml (20 μl) of blood in 0.38 ml of lysing fluid.
in a 75 × 10 mm plastic (or glass) tube. The diluent is 2% (20 ml/l) acetic acid coloured pale violet with gentian violet. After sealing the tube with a lid, mix the diluted blood in a mechanical mixer or by hand for at least 2 min by tilting the tube to an angle of about 120° combined with rotation, thus allowing the air bubble to aid in mixing the suspension. Fill a clean, dry counting chamber, with its coverglass already in position, without delay. This is more easily accomplished with the aid of a plastic Pasteur pipette or a capillary glass tube that has been allowed to take up the suspension by capillarity. Take care that the counting chamber is filled in one action and that no fluid flows into the surrounding moat.Leave the chamber undisturbed on a bench for at least 2 min for the cells to settle, but not much longer, because drying at the edges of the preparation initiates currents that cause movement of the cells after they have settled. The bench must be free of vibrations, and the chamber must not be exposed to draughts or to direct sunlight or other sources of heat. It is important that the coverglass should be of a special thick glass and perfectly flat, so that when laid on the counting chamber, diffraction rings are seen. The coverglass should be of such a size that when placed correctly on the counting chamber, the central ruled areas lie in the centre of the rectangle to be filled with the cell suspension.
If any of the following filling defects occur, the preparation must be discarded and the filling procedure must be repeated using another clean dry chamber:
- •
Overflow into moat
- •
Chamber area incompletely filled
- •
Air bubbles anywhere in the chamber area
- •
Any debris in the chamber area.
To obtain a coefficient of variation of 5%, it is necessary to count about 400 cells ( Table 26-1 ); in practice, it is reasonable to count 100 white cells. This can be achieved by counting the cells in the four larger corner squares. To minimise distribution errors, count the cells in the entire ruled area (i.e. 9 × 0.1 μl areas in an Improved Neubauer counting chamber).
| No. of Areas | Total No. of Cells Counted (λ) | <SPAN role=presentation tabIndex=0 id=MathJax-Element-1-Frame class=MathJax style="POSITION: relative" data-mathml='λ’>λ√λ λ | Count Variance (σ = 0.92 x <SPAN role=presentation tabIndex=0 id=MathJax-Element-2-Frame class=MathJax style="POSITION: relative" data-mathml='λ’>λ√λ λ ) | Uncertainty of Total Count (λ +/- σ) | Range Per 1 mm 2 Area | Calculated Count/μl |
|---|---|---|---|---|---|---|
| 1 | 50 | 7.1 | 6.5 | 36–64 | 36–64 | 7.2–12.8 |
| 2 | 100 | 10.0 | 9.2 | 91–109 | 40–60 | 8.0–12.0 |
| 4 | 200 | 14.1 | 13.0 | 187–213 | 43–57 | 8.6–11.4 |
| 6 | 300 | 17.3 | 15.9 | 284–316 | 44–56 | 8.8–11.2 |
| 8 | 400 | 20.0 | 18.4 | 382–418 | 45–55 | 9.0–11.0 |
| 10 | 500 | 22.4 | 20.6 | 479–521 | 46–54 | 9.1–10.9 |
| 16 | 800 | 28.3 | 26.0 | 774–826 | 46–54 | 9.3–10.7 |
| 20 | 1000 | 31.6 | 29.1 | 891–1029 | 47–53 | 9.4–10.6 |
| 30 | 1500 | 38.7 | 35.6 | 1464–1536 | 47–53 | 9.5–10.5 |
| 200 | 10 000 | 100.0 | 92.0 | 9908–10 092 | 49–51 | 9.8–10.2 |
λ
where λ = number of cells actually counted and variance is expressed as a percentage. Ideally count data should be analysed assuming a negative binomial distribution, but a simplified method is to apply a reduction factor of 0.92 to adjust for some clustering of cells within the counting chamber. The uncertainty of the count is in the range λ ± σ. In this theoretical example the final (calculated) count is based on the number of cells in a 1 mm 2 area at a dilution of 1:20. There were approximately 50 cells in each 1 mm 2 area. For convenience, results have been rounded to the nearest whole number. Counting in only one or two fields results in a wide variance that is reduced as more cells are counted. However, high precision is achieved only when thousands of cells are counted, which is only possible with automated cell counters.
Calculation
White blood cell count per litre (WBC)
= No . of cells counted × Dilution × 10 6 Volume counted μ l
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree