Abstract
The embryonic development and adult function of the mammalian reproductive tract is greatly influenced by multiple conserved growth factor signaling pathways. These include transforming growth factor-β (TGF-β), wingless-type MMTV integration site (WNT), Hedgehog, epidermal growth factor (EGF), insulin-like growth factor (IGF), phosphatidylinositol 3-kinase (PI3K), Hippo, and Notch. This chapter discusses how these pathways regulate the reproductive tract from its very earliest stages (primordial germ cell formation) through ovarian or testicular differentiation, ovarian folliculogenesis and ovulation, and uterine development and function during early pregnancy. We also focus on the varied roles of these pathways in the onset and progression of reproductive diseases.
Keywords
Fertility, mammalian reproductive tract, TGF-β, folliculogenesis, müllerian duct, embryo implantation, pregnancy
Beginning in early embryonic development, multiple growth factor signaling pathways are essential for viability. In particular, the transforming growth factor-β (TGF-β) family signaling pathways are important in growth and differentiation of a number of cell types, as well as for regulation of embryonic stem cells (ESCs). For example, activins maintain human ESCs in their totipotent state, whereas bone morphogenetic proteins (BMPs) cause differentiation of human ESCs. Throughout reproduction, TGF-β family members function along with other growth factor signaling cascades (e.g., wingless-type MMTV integration site [WNT], epidermal growth factor [EGF], steel-factor receptor [KIT], phosphatidylinositol 3-kinase [PI3K], and Hippo) to permit the maintenance of the germline, development of the reproductive tracts, production of gametes, and creation of the next generation ( Fig. 6.1 ). In this chapter, we focus on major growth factor signaling in the mammalian reproductive tract beginning with the development of primordial germ cells (PGCs), and we primarily concentrate on the role of the TGF-β family with particular emphasis on female reproductive biology. The discussion of growth factors in this chapter is relevant to topics covered in Chapter 8 , Chapter 9 , Chapter 11 , Chapter 12 , Chapter 16 that include the ovarian life cycle, the uterus, implantation, development of the reproductive tract, and male reproduction.
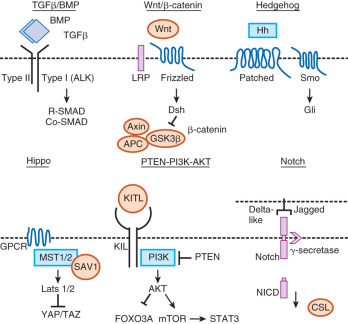
Ovary Development
- ◆
BMP-regulated gene expression is required for PGC formation, proliferation, and migration to the genital ridge.
- ◆
The WNT/β-catenin signaling pathway is essential for ovarian differentiation and development.
- ◆
Multiple signaling pathways such as kit ligand (KITL), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), Notch-Jagged, anti-müllerian hormone (AMH), mechanistic target of rampamycin (mTOR), and signal transducer and activator (STAT) regulate germ cell cyst breakdown to form the “ovarian reserve” of primordial follicles that determines female reproductive lifespan.
Growth Factors in Primordial Germ Cell Formation and Migration
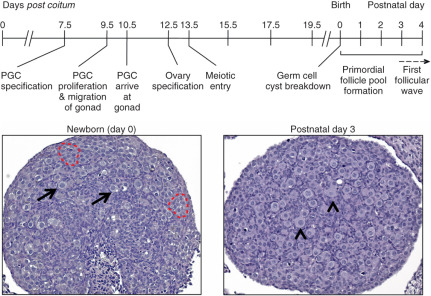
Future components of the mammalian ovary and testis develop long before a distinct bipotential organ can be discerned ( Fig. 6.2 ). Growth factors are key for maintenance of the germline and their migration to the genital ridge following their specification outside the embryo proper. At the onset of gastrulation, PGC progenitors are induced in the epiblast in response to instructive signals from BMPs. This has been defined with great help with the development of mouse genetic models (summarized in Table 6.1 ). BMP4 and BMP8B secreted from the extraembryonic ectoderm, and BMP2 secreted from the visceral endoderm, are required for the early discrimination of PGC precursors from somatic cells of the embryo. These BMPs signal in a dosage-dependent manner to the epiblast cells through a BMP receptor serine-threonine kinase cascade that involves phosphorylation of the SMAD transcription factors, SMAD1 and SMAD5. These SMADs, along with the common SMAD partner, SMAD4, have been shown to function in PGC specification. BMP2, BMP4, and BMP8B act on the pluripotent epiblast cells in the posterior of the mouse embryo between embryonic day (E)5.5 to E6.0 to induce their competency to become PGC precursors. At approximately E6.25, six epiblast cells adjacent to the extraembryonic ectoderm express the BMP-regulated and PGC specification genes, PR-domain containing-1 ( Prdm1; also called Blimp1 ), and Prdm14 , and they commit to becoming true PGC precursors, the first such fate-committed cells of the embryo. By E7.5, approximately 40 founder PGCs are established.
| Gene Symbol | Phenotype/Findings |
|---|---|
| Bmp2 | Embryonic lethal; reduced PGC |
| Bmp4 | Embryonic lethal; no PGC |
| Bmp8b | Viable; male infertility; reduced PGC |
| Smad1 | Embryonic lethal; reduced PGC |
| Smad5 | Embryonic lethal; reduced PGC |
| Smad4 | Embryonic lethal; absent PGC |
| Prdm1 (Blimp1) | Embryonic lethal; PGC specification defect |
| Prdm14 | Infertility; PGC specification defect |
| Pou5f1 (Oct4) | Pluripotency marker |
| Nanog | Pluripotency marker |
| Sox2 | Pluripotency marker |
| Kitl | Variable phenotypes depending on mutation; PGC migration defect |
| Kit | Variable phenotypes depending on mutation; PGC migration defect |
| Tgfbr1 (ALK5) | Embryonic lethal; enhanced PGC migration |
Once PGC specification has occurred at E7.5 in the mouse embryo, PGCs express markers of pluripotency including POU (Pit-Oct-Unc) domain class 5 transcription factor 1 ( Pou5f1 ; also called Oct4 ), sex determining region Y-box 2 (Sox2) , Nanog , as well as developmental pluripotency-associated-3 ( Dppa3 ; also called Stella ) and alkaline phosphatase (Alpl). Between E7.5 and E8.5, the PGCs demonstrate major chromatin changes, increasing the levels of trimethylation of lysine 27 on histone H3 (H3K27me3) and erasing the dimethylation (H3K9me2) marks, which are patterns that resemble the chromatin patterns of pluripotent stem cells. The H3K27me3 marks appear to repress the somatic cell gene expression program similar to ESCs. These changes cause PGC arrest at the G2 stage of the cell cycle, transiently causing the PGCs to become transcriptionally silent as they migrate from the base of the yolk sac along the hindgut to the genital ridge.
An extracellular matrix gradient as well as various chemoattractants are important for appropriate PGC migration. If too much extracellular matrix is deposited, PGCs show reduced migration. For example, suppression of TGF-β signaling by genetically removing the TGF-β type I receptor, Tgfbr1, leads to enhanced migration due to reduction in the levels of TGF-β-induced collagen type 1 in the extracellular matrix. Alternatively, in vitro migration assays demonstrate that KITL, a growth factor that binds to KIT on PGCs, functions as an effective chemoattractant for PGC migration into the genital ridge. Consistent with the pleiotropic roles of the KITL/KIT pathway, these proteins not only function in PGC migration, but also aid in PGC survival and proliferation. The PI3K/AKT and SRC kinase pathways are also involved downstream of KIT in the functioning of the PGCs.
Ovary Specification
PGCs proliferate during their migration to the genital ridge and after colonization. At this time, the gonad is not sexually differentiated and is termed bipotential or indifferent . BMP signaling within the bipotential gonad is necessary to develop a niche for migrating PGCs. Deletion of the BMP type I receptor, Bmpr2a/Alk3 , in the genital ridge leads to death of somatic cells within the mesonephric mesenchyme and reduced levels of Kitl in the coelomic epithelium, ultimately decreasing the number and migration of the PGCs. In mice, PGC proliferation ceases around E10.5 to 11.5, corresponding to 8 to 9 weeks of gestation in humans. Bmp7 is required for germ cell proliferation and Bmp7 null mice have reduced numbers of germ cells after E11.5. Activin may substitute for BMP7 in human embryos, since BMP7 is not expressed in the human ovary and BMP signaling alternatively promotes germ cell apoptosis. Activin increases germ cell survival proliferation in human ovaries prior to the entry of PGCs into meiosis.
Differentiation of the bipotential gonad to form an ovary requires multiple signaling inputs, including the WNT/β-catenin pathway. Partial sex reversal is seen in XX mice null for Wnt4, the WNT pathway activator protein R-spondin 1 (Rspo1) , or follistatin (Fst) , an antagonist of activin and BMP signaling. Loss-of-function RSPO1 in XX patients results in complete female-to-male sex reversal while duplication of the chromosome region containing WNT4 and RSPO1 causes XY male-to female-sex reversal. Similarly, XY male-to-female sex reversal is found in mice with a stable overexpression of β-catenin. β-catenin appears to be a central component to ovary specification, integrating signaling from the WNT and TGF-β family. Activation of WNT signaling allows stabilization and nuclear translocation of β-catenin, which interacts with a number of transcription factors of the TCF/LEF family to promote specific gene transcription. Increased levels of β-catenin prevent the expression of SOX9, a transcription factor that promotes testes development by inducing Sertoli cell differentiation. β-catenin also increases expression of Fst , which shows a female-specific expression pattern at E11.5. Wnt4 -null or Fst -null ovaries develop a coelomic vessel similar to that found in testes, and germ cells are lost by birth. Wnt4 -null ovaries show an upregulation of Inhbb (activin B) and Fst -null ovaries also express Inhbb . Genetic crosses between Wnt4 or Fst -null mice to Inhbb null mice prevents development of the coelomic vessel and rescues normal ovary development, indicating that Inhbb expression (via suppression by Wnt4) or activity (via antagonism through follistatin) must be limited to maintain ovary specification. Bmp2 is also expressed in a female-specific pattern after E11.5, although Bmp2 -null mice die prior to this time period, and its function in the developing ovary has yet to be determined.
Formation of the Ovarian Reserve
During embryonic development, oocytes within the ovary are found as clusters, which are known as germ cell cysts (see Fig. 6.2 ). These clusters form by both aggregation and clonal division. Meiotic progression in oocytes ceases at the diplotene stage of prophase I when germ cells are enclosed by somatic cells into individual follicles called primordial follicles. Oocytes in primordial follicles remain arrested until released to complete meiosis I during ovulation and form the “ovarian reserve,” which is thought to ultimately determine reproductive lifespan. Germ cell cyst breakdown occurs around birth in mice or during the second trimester in humans. In mice, the majority of germ cells within the cysts will die, with an estimated one-third remaining as primordial follicles. Massive germ cell loss also occurs in humans, with an estimated 1 million oocytes surviving at birth from approximately 6 million in the fetal human ovary.
The mechanism for how oocytes become enclosed in primordial follicles has remained elusive, although multiple signaling inputs are likely required, particularly from those factors that mediate the interaction of oocytes with germ cells ( Fig. 6.3 ). Signaling pathways that have been implicated in germ cell cyst breakdown are KITL, NGF, BDNF, Notch-Jagged, AMH, mTOR, and STAT. One mechanism for primordial follicle formation shows that Rac1, a Rho GTPase, promotes germ cell cyst breakdown by inducing nuclear translocation of signal transducer and activator of transcription 3 (STAT3) to activate transcription of oocyte-specific genes including Jagged1 , Nobox , Gdf9 , and Bmp15 in cultured mouse ovaries. Another mechanism suggests mTOR signaling regulates KITL to facilitate germ cell nest breakdown and subsequent primordial follicle assembly. Genetic ablation of Tsc1 or Tsc2 , negative regulators of mTOR complex 1 (mTORC1), in oocytes impairs the differentiation of pregranulosa cells and causes premature primordial follicle activation and follicular depletion by early adulthood. Suppression of mTORC1 is critical for maintaining the pool of follicles within the ovarian reserve. Treatment of somatic cells with mTOR inhibitors impedes somatic cell invasion into germ cell cysts in in vitro cultured ovaries. Therefore mTOR signaling is critical for bidirectional communication between somatic cells and oocytes to facilitate germ cell cyst break down and follicle assembly. Improper germ cell cysts breakdown can manifest as multiple oocytes within follicles (called polyovular follicles ), although their biologic effects on fertility are not certain. Mice with mutations in the TGF-β family, such as ovarian activin βA (Inhba) deficiency, inhibin alpha subunit overexpression, and knockout mice for oocyte-expressed Bmp15 , often show increases in polyovular formation. In addition, polyovular follicle formation can be generated by exposure of neonatal mice to estrogen and estrogen-like compounds, which may suppress ovarian activin expression, thus disrupting the regulation of germ cell cyst breakdown.

The origin of the somatic cells that surround each primordial follicle and eventually become granulosa cells of the follicle has been controversial. Sertoli cells and granulosa cells may arise from ingressing cells of the coelomic epithelium during gonad development, although the timing of their specification may be different. In the testis, regulation of the transcription factor Sox9 by the testis-determining factor SRY is required for Sertoli cell differentiation. In the ovary, forkhead box 2 (Foxl2) acts to maintain granulosa cell differentiation, in part by suppressing the expression of Sox9 . Mutations in the FOXL2 genes are associated with several human diseases. The FOXL2 gene is mutated in women with blepharophimosis ptosis epicanthus syndrome (BPES), which in one form is associated with primary ovarian failure. Mice null for Foxl2 are sterile with small ovaries and defects in primordial follicle assembly and growth ( Fig. 6.4 ). FOXL2 interacts with different transcription factors, including SMAD2 and SMAD3, to control gene transcription in nonovarian cell types, such as regulating FSHβ , FST , and gonadotropin releasing hormone receptor synthesis in anterior pituitary cells. In the developing mouse ovary, BMP2 and FOXL2 also cooperatively upregulate Fst gene expression. A somatic missense mutation in FOXL2 is associated with almost all adult granulosa cell tumors, a rare class of ovarian tumors. This mutation causes an amino acid substitution in the DNA-binding domain of FOXL2, although its functional consequence has not been fully clarified.
Ovarian Folliculogenesis
- ◆
TGF-β superfamily members, GDF9, BMP15, AMH, activin, and inhibin function to regulate ovarian follicle growth and development.
- ◆
Oocyte-secreted growth factors (GDF9 and BMP15) and EGF-like family members (amphiregulin, epiregulin, and betacellulin) modulate cumulus cell expansion and oocyte maturation.
Ovarian Follicle Development
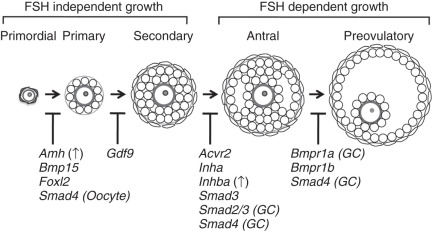
Once formed, quiescent primordial follicles are currently thought to supply all of the oocytes during the reproductive lifespan of the female. This source of oocytes appears to be nonrenewable in mammals, although germlike stem cells that can be differentiated into oocytes in culture have been reported. Following their recruitment, the cohort of primordial follicles undergoes a defined number of developmental follicular stages, the earliest of which are independent of input from pituitary gonadotropins (see Fig. 6.4 ). The PI3K is a key pathway within oocytes for their activation within primordial follicles. Activation of PI3K signaling through deletion of the negative regulatory protein, Pten , or loss of an inhibitory transcription factor, Foxo3a , results in global activation of primordial follicles and sterility in mice. The requirement of PI3K signaling in oocytes may be limited to the primordial follicle stage, because conditional deletion in mice of Pten from the primary stage onward has no effect on reproduction. Other oocyte-specific transcription factor cascades regulate early oocyte development, including Nobox , Sohlh1 , Sohlh2 , and Figla, which initiate an oocyte-specific gene expression pattern. Mutations in NOBOX and FIGLA , or their downstream targets, such as GDF9 and BMP15 , have been further implicated in the development of primary ovarian insufficiency in women.
Following primordial follicle activation, pregranulosa cells undergo a squamous to cuboidal transition, forming the “primary follicles” (see Fig. 6.4 ). Granulosa cell proliferation continues, and a second somatic cell layer composed of thecal cells recruited from the ovarian stroma, forms adjacent to the basement membrane surrounding the outermost layer of granulosa cells, resulting in a population of “secondary follicles.” The oocyte-specific TGF-β family member, growth, and differentiation factor 9 (Gdf9) are required at this stage, as well as mice null for Gdf9 arrest at the primary to secondary stage and do not develop a theca layer (see Fig. 6.4 ; Table 6.2 ). Thecal cells are required for steroidogenesis, as they produce androgens necessary for production of estrogen, and they may provide additional growth factors and nutrients necessary for follicle growth. The follicle arrest in Gdf9 -null follicles is due to overproduction of inhibin α (Inha) , one of the subunits required to make the protein hormone inhibin, an antagonist of activin. Ovaries from Inha-Gdf9 double knockout (DKO) mice develop multilayer follicles and form a morphologically distinct layer of cells similar to thecal cells. However, the presumptive thecal cells in Inha-Gdf9 DKO ovaries never fully differentiate and are nonfunctional due to lack of expression of key genes, such as 17 alpha-hydroxylase (Cyp17), the luteinizing hormone (LH) receptor (Lhcgr), and Kit .
| Mutant Gene | Reproductive Phenotype | Fertility Status |
|---|---|---|
| Amh | Increased primordial follicle activation | Normal |
| Bmp6 | Reduced ovulation | Subfertile |
| Bmp7 | Reduced primordial germ cells | N.A. |
| Bmp15 | Decreased ovulation and fertilization rates | Normal to subfertile |
| Gdf9 | Follicular arrest at the primary follicle stage | Infertile |
| Fst | Loss of oocytes by birth | N.A. |
| Fst (Conditional) | Reduced growing follicles, ovulation and fertilization defects | Subfertile to infertile |
| Inha | Infertility, abnormal follicle growth, sex-cord stromal tumors | Infertile |
| Inhba (Conditional) | Increased polyovular follicles | Subfertile |
| Inhbb | Lactation defects | Infertile |
| Inhba Inhbb (Conditional) | Increased numbers of antral follicles and accumulation of corpora lutea | Infertile |
| Tgfb2 | Increased oocytes at birth | N.A. |
An additional TGF-β family member, AMH, is also important as a regulator of early follicle development, particularly for inhibition of primordial follicle activation (see Table 6.2 ). AMH was discovered based on its function in sex determination and its ability to cause regression of the müllerian duct during male reproductive tract development (see the later section “ Regression of the müllerian ducts ”). In the ovary, AMH is expressed from granulosa cells of growing follicles until their development becomes dependent upon follicle stimulating hormone at the later antral stages. Mice null for AMH initially have normal-sized litters compared to wild-type mice but demonstrate an accelerated loss of primordial follicles associated with an increasing number of growing follicles. This suggests that AMH negatively regulates primordial follicle activation. In women, serum levels of AMH correlate with the number of antral follicles, and both decline with age. Thus, AMH has become a useful clinical marker of ovarian reserve and reproductive disease in women. Its clinical utility has been expanding in scope for use in monitoring ovarian reserve, response to in vitro fertilization protocols, granulosa cell tumor recurrence, and diagnosis of ovarian diseases such as primary ovarian insufficiency and polycystic ovary syndrome (PCOS).
Coordination of growth and communication between cell types in the follicle is essential for follicle development to occur. Multiple signaling pathways have critical inputs, including WNT-β-catenin, steroid hormone signaling, and KIT-KITL. Preantral follicle growth is controlled largely via intraovarian factors independent of pituitary follicle-stimulating hormone (FSH) (see Fig. 6.4 ). Mice null for the FSHβ subunit (Fshb) are infertile and do not form antral follicles. A similar block in follicle development is also seen in mice null for the activin binding receptor, Acvr2a , which have suppressed FSH production due to loss of activin signaling in the pituitary. Exogenous administration of gonadotropins in Fshb -null females results in ovulation, thus “rescuing” the phenotype, and demonstrates the switch from gonadotropin-independence to gonadotropin-dependence at the final stages of follicle growth (see Fig. 6.4 ).
Recent studies have additionally implicated signaling by the Hippo pathway during folliculogenesis (see Fig. 6.1 ). The Hippo pathway is a developmentally conserved pathway, first discovered in Drosophila, which controls organ size and cell homeostasis, and has been implicated in human diseases such as cancer. Various components of the pathway, including large tumor suppressor homologues (LATS1, LATS2), mammalian sterile 20-like protein kinase I (MST1), and yes-associated protein (YAP1), are expressed in mouse oocytes of primordial, primary, secondary and antral follicles, as well as in somatic granulosa cells. The expression of these components is stage-specific and dependent upon mouse age. Lats1 null ovaries undergo an increase in germ cell death and contain fewer primordial follicles and activated follicles compared to wild-type ovaries. Mutations in YAP are associated with PCOS, a disease of anovulation, hyperandrogenism, and abnormal ovarian follicle growth. Furthermore, ovaries from women with primary ovarian insufficiency (POI) showed preantral follicle growth following fragmentation to abrogate Hippo signaling and treatment with PTEN inhibitors and PI3K activators to stimulate the AKT pathway after grafting. Mature oocytes from fragmented grafts were obtained for in vitro fertilization-embryo transfer (IVF-ET) and the procedure successfully resulted in the birth of a healthy baby. Collectively, these findings establish that the Hippo signaling pathway restricts follicle growth and may provide a useful alternative fertility treatment for POI patients or women struggling to become pregnant due to other conditions and who may not be suitable for standard IVF.
Activin is a homodimer of β subunits derived from one of four genes in mammals, primarily Inhba or Inhbb, which generate activin A (βA:βA homodimers), activin B (βB:βB), or activin AB (βA:βB). Inhibin is an antagonist of activin and is formed by the dimerization of the Inhba or Inhbb gene products with a dissimilar alpha subunit, a product of the Inha gene (α:βA, inhibin A or α:βB forming inhibin B). Activin and inhibin were discovered based on their ability to stimulate (activin) or inhibit (inhibin) the secretion of FSH from the pituitary. They are now known to be potent regulatory proteins with paracrine, autocrine, and endocrine functions in embryonic and adult tissue homeostasis, including the ovary. Inhibin antagonizes activin by competition for activin’s binding receptors in conjunction with betaglycan, a coreceptor. Activin signaling can also be inhibited by follistatin, a high-affinity binding protein that prevents activin from binding to its receptors.
The activin subunits Inhba and Inhbb are expressed primarily in granulosa cells, although the receptors localize to thecal cells, granulosa cells, and oocytes. Intraovarian activin promotes cell proliferation, and may modulate FSH action and steroidogenesis. Inhba -null mice and Fst- null mice die shortly after birth with craniofacial defects. Inhbb -null female mice give birth to normal-sized litters, but offspring fail to thrive because the mothers have lactation defects. Tissue-specific (“conditional”) knockouts of Inhba and Fst have been generated in granulosa cells ( Table 6.3 ; see also Table 6.2 ). Stepwise removal of both activin subunit genes (i.e., conditional deletion of Inhba concurrent with global loss of Inhbb ) results in female sterility. Activin-deficient mice have increased antral follicles, but defective ovulation, suggesting that activin’s role at the preantral stage is either limited or redundant with other factors. Strikingly, activin-deficient ovaries display an increasing number of corpora lutea with age and increases in two hormones of the reproductive axis, FSH, and progesterone. Corpora lutea, the temporary endocrine structures that produce progesterone necessary for pregnancy to occur, fail to regress in activin-deficient ovaries, supporting a role for activin in the very terminal stage of follicle development. One key gene that is regulated by the TGF-β family, Ctgf (connective tissue growth factor), is downregulated in activin-deficient ovaries. Conditional deletion of Ctgf also results in impaired ovulation and increases the number of corpora lutea as the activin-deficient ovary. Further evidence suggests that FOXO1/3 interact with the activin and BMP2 pathways in granulosa cells to regulate pituitary FSH production and promote follicle maturation at the preantral or early antral stage.
| Gene | Cre | Cell-Specific Deletion | Reproductive Phenotype | Fertility Status |
|---|---|---|---|---|
| Smad1 | Amhr2cre | Granulosa cell | None | Normal |
| Smad2 | Amhr2cre | Granulosa cell | None | Normal |
| Smad3 | Amhr2cre | Granulosa cell | None | Normal |
| Smad4 | Amhr2cre | Granulosa cell | Reduced antral follicles and ovulation rates, cumulus cell defects, increased luteinization, increased progesterone | Subfertile, then 50% infertile by 6 months |
| Smad4 | Gdf9-icre | Oocyte | Reduced litter sizes | Subfertile |
| Smad4 | Zp3-cre | Oocyte | — | Normal |
| Smad5 | Amhr2cre | Granulosa cell | None | Normal |
| Smad8 | Knockout | Global | None | Normal |
| Smad2 Smad3 | Amhr2cre | Granulosa cell | Reduced antral follicles and ovulation rates, cumulus cell defects | Subfertile, then infertile at 5 months |
| Smad1 Smad8 | Amhr2cre | Granulosa cell | None | Normal |
| Smad5 Smad | Amhr2cre | Granulosa cell | None | Normal |
| Smad1 Smad5 | Amhr2cre | Granulosa cell | Granulosa cell tumors | Subfertile, then infertile at 4–6 months |
| Smad1 Smad5 Smad8 | Amhr2cre | Granulosa cell | Granulosa cell tumors | Subfertile, then infertile at 4–6 months |
| Smad 1 Smad5 Smad4 | Amhr2cre | Granulosa cell | Increased follicular atresia, reduced granulosa cell tumor size and metastasis | Sterile |
Follistatin and inhibin are two antagonists of activin. Conditional deletion of follistatin in granulosa cells results in infertility in female mice (see Table 6.2 ). Deletion of the inhibin alpha subunit (Inha) also results in infertility and defects in follicle growth, but importantly causes the development of sex-cord stromal tumors (see Table 6.2 ). Activin-production from these tumors causes a cancer cachexia-like death in early adult Inha -null male and female mice. Tumor development in Inha -null females can be slowed by removing Acvr2a , deleting one of activin’s downstream transcription factors ( Smad3 ; see later), or by overexpressing follistatin. These models support a growth-promoting role for activin in granulosa cells and sex-cord stromal tumor development.
SMAD2 and SMAD3 are the principal transcription factors that relay activin, TGF-β, and GDF9 signaling. Significant functional redundancy between these proteins likely exists during granulosa cell development, because single conditional knockouts of either Smad2 or Smad3 do not show reproductive defects (see Table 6.3 ). However, Smad2 Smad3 conditional DKO mice have reduced female fertility and fecundity and reduced numbers of antral-stage follicles and follicles that had undergone premature luteinization. Furthermore, cumulus expansion, a process of extracellular matrix formation and loss of oocyte-cumulus cell connections that occurs during ovulation, is altered (see below). The phenotype of the Smad2 Smad3 DKO is similar to that found for granulosa cell-specific Smad4 conditional knockouts, although the latter also showed increased atretic preantral follicles, suggesting that a SMAD4-dependent non-SMAD2/3 pathway may be important for preventing atresia in the preantral follicle stage.
Several BMPs are expressed during preantral and antral stage follicle development from oocytes ( Bmp6 and Bmp15 ), granulosa cells (Bmp2), and thecal cells ( Bmp4 and Bmp7 ). Bmp6 -null mice have mild changes in their fecundity, and BMP6 may regulate some aspects of ovulation. BMP15 plays critical roles in sheep fecundity (see later section) and may interact with GDF9, as mice null for Bmp15 but containing one allele of Gdf9 have reduced litter sizes compared to Bmp15 -null mice. Conditional deletion in the ovary of both of the BMP receptors, Bmpr1a and Bmpr1b, results in granulosa cell tumor development, as does deletion of the downstream BMP signaling SMADs, Smad1 and Smad5 . The histologic, hormone, and gene expression profile of the Smad1 Smad5 DKO mice is similar to the juvenile form of human granulosa cell tumors. Smad1 Smad5 DKO mice die from metastatic disease but do not develop the cancer cachexia-like syndrome of Inha -null mice, suggesting that activin is not overexpressed from the Smad1 Smad5 DKO tumors. However, activated phosphorylated SMAD2 and SMAD3 is found in Smad1 Smad5 DKO or Inha null tumors, as well as human juvenile granulosa cell tumors samples, suggesting that an active SMAD2/3 pathway plays a conserved role in development or progression of granulosa cell tumors. Codeletion of Smad4 in Smad1 Smad5 DKO mice (creating a triple Smad1 Smad5 Smad4 conditional KO) results in slowing growing granulosa cell tumors with increased cellular apoptosis, supporting the hypothesis that a SMAD4-dependent signal, likely through TGF-β signaling, is important in promoting tumor progression.
Cumulus Expansion and Ovulation
Preantral follicle growth is followed by an antral follicle stage, which is defined by the presence of a fluid fill cavity, the antrum (see Fig. 6.4 ). Under the influence of pituitary gonadotropins, antral follicles are selected to undergo final differentiation, ovulation, and, subsequently, corpus luteum formation. Because of the presence of an antrum, preovulatory follicles contain two independent populations of granulosa cells: mural granulosa cells line the follicle wall and are closest to the theca, basement membrane, and vasculature, while cumulus cells surround the oocyte and are linked initially with the oocyte for their functions ( Fig. 6.5 ). The growth factor contributions of the mural and cumulus cell and their relevance to ovulation are most apparent at the time of the LH surge. The LH receptor (Lhcgr) is preferentially expressed only in mural granulosa cells, allowing them to respond to the LH surge, which initiates a cascade of events leading to oocyte meiotic resumption, cumulus expansion, follicle rupture, and terminal differentiation of the remaining granulosa and thecal cells to create the corpus luteum. Cumulus cell expansion, which is the production of a hyaluronan-rich extracellular matrix surrounding the oocyte, is initiated by the LH surge and is required for normal ovulation and fertilization. However, since the cumulus granulosa cells lack LHCGR and yet respond to the LH, this indicates an indirect effect of the LH surge on their fate.
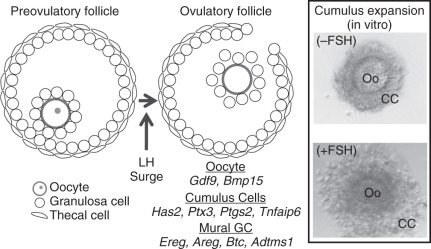
How does the LH surge lead to induction of target genes in cumulus cells that are critical for cumulus expansion? Conti and colleagues have shown that the LH surge causes a rapid increase in EGF-like family members, amphiregulin (AREG), epiregulin, and betacellulin (BTC) specifically in mural granulosa cells of preovulatory follicles (see Fig. 6.5 ). These ligands are synthesized as membrane proteins and released from the cell surface into the antral fluid by proteolytic cleavage. These EGF-like proteins activate the EGF receptor (EGFR) on cumulus cells to exert the indirect effect of the LH surge to stimulate cumulus expansion and oocyte maturation. The effects of these EGF-like factors on cumulus expansion occur through upregulation of the genes encoding hyaluronan synthase 2 (Has2), tumor necrosis factor-induced protein 6 (Tnfaip6), pentraxin 3 (Ptx3), and prostaglandin synthase 2 (Ptgs2), whose products are essential for formation and stabilization of the extracellular matrix of the cumulus cells (see below). Transcripts for Areg , Ereg , and Btc are also detected in cumulus oocyte complexes after the surge, suggesting that an autocrine regulatory loop maintains high EGF-like growth factor expression in cumulus cells. These data are not only relevant to mice, since amphiregulin is abundant in human follicular fluid from patients undergoing in vitro fertilization.
Although EGF-like factors play an important role in cumulus expansion and oocyte maturation, oocyte-secreted growth factors are also essential for cumulus expansion. In vitro, expansion of isolated cumulus oocyte complexes can occur with the addition of EGF (as well as FSH and cAMP analogues). The oocyte is required for expansion, since oocytectomized cumulus oocyte complexes fail to expand when only FSH or EGF is added to the culture medium. Expansion of OOX complexes are rescued by coculture with denuded oocytes or conditioned medium from denuded oocytes, indicating the presence of oocyte growth factors is also involved in the expansion. As mentioned earlier, follicular development in Gdf9 KO mice is arrested at the primary follicle stage. However, recombinant GDF9 upregulates Has2 , Ptgs2 , Ptger2 , Tnfaip6 , and Ptx3 in granulosa cell culture systems. Injection of mouse oocytes with Gdf9 small interfering (si)-RNA, but not Bmp15 si-RNA, suppresses Has2 and Ptgs2 expression, and cumulus expansion when oocytectomized cumulus complexes are incubated with knockdown oocytes.
The cumulus cell matrix contains an essential meshwork of hyaluronan and proteins. Hyaluronan chains from the structural backbone of the cumulus matrix, and these chains are stabilized through interactions with other matrix proteins. TNFAIP6 catalyzes the formation of covalent cross-links between hyaluronan and the heavy chain of serum-derived inter-α-trypsin inhibitor (IαI), and PTX3 stabilizes the cumulus matrix through interactions with IαI. PTGS2 synthesizes PGE2 and prostaglandin EP2 receptor (PTGER2); it appears to function upstream of TNFAIP6. Although Has2 ovary-specific knockouts have not been generated, the importance of the regulation of the other EGF/GDF9/BMP15 regulated gene products in vivo in cumulus expansion is obvious. Targeted disruption of Ptgs2 , the prostaglandin E receptor 2 (Ptger2) , Tnfaip2 , or Ptx3 results in abnormal or absent cumulus expansion and extreme subfertility while Tnfaip6 -null females are sterile. Knockdown of Has2 in cultured cumulus oocyte complexes suppresses cumulus expansion. IαI biosynthesis in the liver requires alpha-1-microglobulin/bikunin light chain (encoded by Ambp ) for proper assembly and secretion, and Ambp -null females have impaired cumulus expansion and subfertility. Thus oocyte-secreted growth factors (GDF9 and BMP15) and EGF-like growth factors (AREG, EREG, and BTC) combine to induce an array of genes that are necessary for mammalian cumulus expansion and fertility.
Mutations in mice, sheep, and humans have been important in identifying key functions of GDF9, BMP15, and their downstream gene products in cumulus expansion. GDF9 signals through a heterodimeric complex of type 1 and a type 2 receptors to phosphorylate SMAD2/3 and complex with SMAD4 and conditional deletion of both Smad2 and Smad3 or Smad4 in granulosa cells using Amhr2-cre disrupts cumulus expansion in vivo and suppresses fertility (as mentioned earlier). Recent studies demonstrated that recombinant mouse and human GDF9:BMP15 heterodimers are more bioactive than GDF9 and BMP15 homodimers in cumulus expansion in vitro . Furthermore, GDF9:BMP15 heterodimers predominantly activate the SMAD2/3 pathway likely through a BMPR2-ALK4-ALK6 receptor complex in mouse and human granulosa cells. Naturally occurring mutations in sheep BMP15 and GDF9 or the BMP type I receptor, BMPR1B/ALK6 , modify ovulation rate in sheep ( Table 6.4 ). In most cases, heterozygous mutations in BMP15 or GDF9 increase ovulation rates, while homozygous null animals are sterile. Ovaries from homozygous mutant sheep appear similar to mouse Gdf9- null ovaries, with follicles arrested at the primary stage. Partial immuno-neutralization against GDF9, BMP15, or in combination in sheep will increase ovulation rate and number of lambs, indicating that both GDF9 and BMP15 regulate this process. Genetic variants have been identified in women with premature ovarian failure ( BMP15 or GDF9 ) or dizygotic twinning (GDF9).
| Gene | Mutation | Breeds | Protein Domain | Mutation Type |
|---|---|---|---|---|
| GDF9 | FecG H | Belclare, Cambridge | Mature | Substitution |
| FecG E | Santa Inês * | Mature | Substitution | |
| BMP15 | FecX B | Belclare | Mature | Substitution |
| FecX I | Inverdale | Mature | Substitution | |
| FecX H | Hanna | Mature | Premature stop | |
| FecX G | Galway | Prepro | Substitution; premature stop | |
| FecX L | Lacaune | Mature | Substitution | |
| FecX R | Rasa Aragonesa | — | Premature stop | |
| BMPR1B | FecB | Booroola † , Merlino, Hu, Han, Garole, Javenese Thin-tail | Kinase domain | Substitution |
* Heterozygotes have normal ovulation rates, but rates increase in homozygous sheep.
† Heterozygotes and homozygotes have increased ovulation rates with the heterozygotes midway between homozygotes and noncarriers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree