Growth and Development of the Musculoskeletal System
Donald E. Sweet†
Thomas V. Smallman
†Dr. Sweet passed away suddenly on August 18, 2004. The Chairman of the Department of Orthopaedic Pathology, Armed Forces Institute of Pathology, Washington, DC, and an educator par excellence, he was in the process of preparing this chapter with me (T.V.S.), and the chapter is dedicated to his memory. Humble, brilliant, and a contributor in many ways to the world of orthopaedics for 35 years, he will be missed.
The process of growth and development involves an ordered sequence of steps that begins in the embryo. Growth follows a logical order of sequential appearance, maturation, and removal of progressively more differentiated structures and tissues. The morphology of this sequence will be demonstrated in this chapter. The genetic mechanisms underlying this process are not fully known; reference will be made, where appropriate, to areas of ongoing investigation.
Emryogenesis
Skeletal Development
The skeleton arises from the mesoderm, the middle cell layer of the three-layer embryo (Table 14-1).
Limb Bud Development
Limb Bud Elements
Central core gives rise to bones and joints.
Around central core: looser tissue from which muscles, tendons, ligaments, and vessels will develop
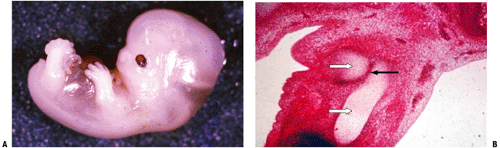
Ectodermal covering with a central condensation of mesoderm (Fig. 14-1)
Table 14-1 Origin of Skeletal Elements According to Mesodermal Site | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Outgrowth
Governed by relationship between the thickened distal ectoderm (apical ectodermal ridge [AER]) and the underlying mesoderm
Extremely rapid reproduction of large numbers of cells in the central core as well as in the ectoderm results in a rapid elongation of the limb bud.
Several growth factors regulate activities at the AER and adjacent zones:
Fibroblast growth factors (FGFs; especially FGF4 and FGF8) control cell differentiation in the immediately adjacent limb bud along its longitudinal axis.
Zone of polarizing activity (ZPA) at posterior distal part of the limb bud controls anterior posterior patterning through the protein sonic hedgehog (Shh).
Innervation
Nerves extend outward from the central nervous system and attach themselves to developing muscles.
Innervation of bone and periosteum is by sympathetic and sensory fibers that contribute to regulation of vascular control and perception of nociceptive stimuli.
Nerves accompany vessels into bone both into the marrow space and into the Haversian and Volkmann canal systems.
Differentiation
Unfolds from proximal to distal in a sequential manner (hip, then femur, then knee, then calf, and so forth; see Fig. 14-1)
Cartilage formation from the mesenchymal condensations begins during the sixth week.
Fundamental Concepts in Skeletal Genesis
To understand the further development of the limb bud, one must consider several fundamental aspects of bone development.
Bone is not created de novo but rather as an applique on or within a pre-existing mesenchymal framework. In skeletal embryogenesis, then, bone forms:
On a collagen framework called woven bone in the craniofacial bones and clavicle, or
On a calcified cartilage framework in the remainder of the skeleton.
Once bone has formed, bone modeling or remodeling involves bone deposition on previously made bone.
Bone is made by osteoblasts and is deleted by osteoclasts. Once formed, the skeleton attempts to maintain homeostasis in the long run by balancing formation and deletion. A surgeon, Julius Wolff, proposed in 1891 that mechanical stress determined the architecture of bone in that bone is made in areas where there is mechanical stress and is deleted where there is little stress. Homeostasis in the skeleton involves a balance among mechanical, metabolic, and circulatory influences acting on the effector cells, osteoblasts and osteoclasts. The discipline of cell biology is now demonstrating that the final pathways directing these cells in these fields of influence involve polypeptide signaling substances, hormones, and their receptor systems.
Normal Bone Formation
In the embryo and throughout life there are three types of normal bone formation.
Membranous (Woven) Bone Formation
In membranous bone formation (Figs. 14-2 and 14-3), bone is formed directly by osteoblasts, the life cycle of which includes:
Osteoblast origin: subset of pluripotent stromal stem cell line
Preosteoblast (flat, spindle cell): inducible osteoprogenitor cells (IOPCs) proliferate and make bone morphogenic protein (BMP) and osteopontin
Molecular regulator of osteoblast differentiation is transcription factor Cbfa1; bone morphogenic proteins 2 and 7 (BMP2, BMP7) regulate Cbfa1 gene expression (Box 14-1).
Proliferating osteoblast (cell is plumper, increasing rough endoplasmic reticulum)
Differentiating osteoblast
Mature osteoblast (cell is cuboidal, looks like a plasma cell) expresses alkaline phosphatase
Osteoblast, matrix mineralizing: most osteoblasts undergo apoptosis, some become osteocytes, some remain on the endosteal surface as bone lining cells
Osteocalcin is expressed by the osteoblast in this stage.
Osteocyte, resident in bone
One in ten osteoblasts does not participate in the extreme activity creating the osteoid seam but becomes a resident osteocyte, responsible for maintaining the bone that has been produced around it.
Cells communicate through dendritic processes and gap junctions, forming a functional syncytium with the bone lining cells.
Life span of an individual osteocyte may be months (fetal bone) to years (adult bone).
Bone surface lining cell (BSLC)
Can either be resting or in an activated state making bone
Resting cells provide cytokine signal to activate bone remodeling.
May adopt fat cell morphology in the aging
All bone in the body is made by osteoblasts (see Fig. 14-3) and is either skeletal or extraskeletal; dysregulation of these precursor cells may manifest clinically in diseases such as fibrodyplasia ossificans progressiva, a genetic disease characterized by the ossification of skeletal muscle and other connective tissues, or in the periarticular heterotopic ossification that can complicate skeletal trauma.
Enchondral (Endochondral) Bone Formation
In endochondral bone formation, bone is formed on a calcified cartilage template.
Calcified cartilage acts as a nidus on which bone is deposited by osteoblasts derived from perivascular stem cells arising from vessels in the adjacent marrow.
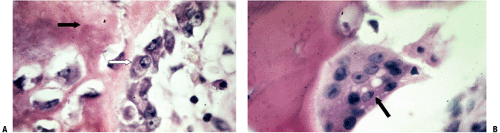
Figure 14-4 is a high-power cut showing a primary trabecula in a growth plate; primary trabeculae anchor the growth plate to the rest of the bone and act as an initial lattice for transmission of mechanical force.
This region undergoes rapid remodeling in the fetus and young and remains the site of most remodeling of bone throughout life.
Primary trabeculae thus remodel rapidly, with removal by osteoclasts of bone and calcified cartilage and replacement by bone, thus forming a secondary trabecula.
Lamellar Bone Formation
In lamellar bone formation, bone is formed on pre-existing bone (Fig. 14-5) in a process that takes days to months to complete.
Lamellar bone consists of layers of collagen, each layer arranged at a different angle from its neighbor, like plywood; this gives mechanical strength to the composite structure. It can be applied to pre-existing woven bone or to lamellar bone.
In woven bone the lamellar bone is applied in sheets as an inlay apposed to the inner surface of the randomly oriented trabecula; the effect is to strengthen the construct.
In lamellar bone the application of new bone is part of the modeling or remodeling process.
Woven bone formation is rapid, and the bone that results is a mechanically weak, open mesh that must be reinforced by a lamellar bone fill-in before significant weight transmission.
Bone Modeling
Definition
Process whereby bone, formed initially in the embryo, is shaped into the adult form as growth occurs
Responsible for the large changes in bone structure during growth
Defining Characteristics
Changes in bone structure occur on existing bone structure.
Bone structure alterations occur by independent action of osteoblasts and osteoclasts.
Bone resorption and formation may occur on different surfaces.
Bone Remodeling
Definition
Process whereby current bone structure is maintained
Cannot cause large changes in bone structure at a given site
Box 14-1 Factors Expressed by the Various Stages of Proliferating and Differentiating Osteoblasts and Their Precursors
BMP 2
BMP 7
Type 1 collagen
Osteopontin
Bone sialoprotein
Alkaline phosphatase
Defining Characteristics
Changes in bone structure occur on or within existing bone structure (same as for modeling).
Osteoblasts and osteoclasts do not act independently but are coupled through cytokine activity and mechanical forces (different than for modeling). Table 14-2 outlines the essential features of osteoblasts and osteoclasts.
Coupled bone deletion and formation at the same site: Intracortical remodeling is affected by the bone remodeling unit (BRU)—teams of osteoclasts excavating a hole in the cortex (10 longitudinal surges of clastic activity over 2 months) followed by osteoblasts filling in the hole (months)
Cancellous bone remodeling occurs at many sites in the honeycomb trabecular array, with activation of bone surface lining cells, recruitment of marrow osteoclasts, creation of a Howship’s lacuna (see Fig. 14-3), and activation of stem cells to become osteoblasts, which fill the defect.
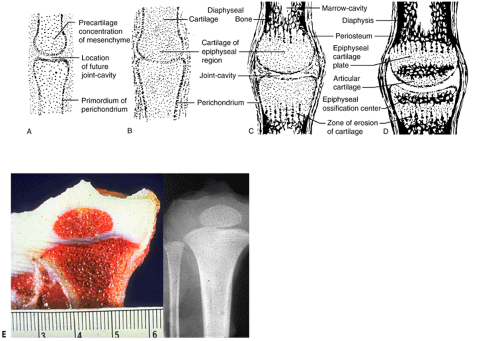
Long Bone Growth
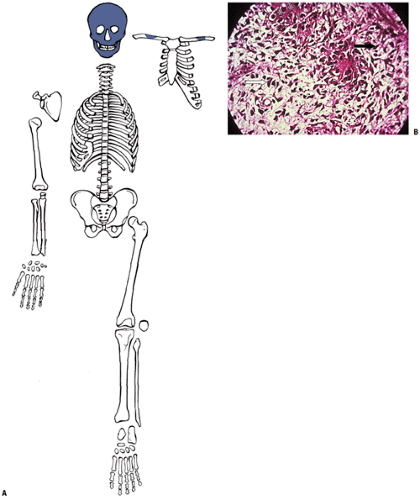
By the ninth week in the embryo the skeleton is preformed, the craniofacial bones as woven bone and the rest as a multiplicity of cartilage models (Fig. 14-6). Subsequent growth of the models progresses in stages.
Stages of Long Bone Development
Before development of ossification centers:
Cartilage models undergo considerable growth in size:
By appositional induction at their surface (“perichondrium”) and
By internal cartilage cell proliferation and maturation
Table 14-2 Essential Features of Osteoblasts and Osteoclasts
Osteoblast (OB)
Osteoclast (OC)
Function
Only bone-forming cell
Only bone-deleting cell
Cell cycle
Stem cell ← pre-OB ← proliferating OB ← mature OB ← mineralizing OB ← apoptotic OB/osteocyte/ bone lining cell
Marrow and circulating OC precursors ← fusion of OC precursors ← multinucleated OC ← apoptotic OC
Cell function
Expression of type I collagen, ALP, osteonectin, osteopontin, bone sialoprotein, biglycan, decorin, matrix Gla-protein, and bone acidic glycoprotein
Attachment to the bone surface ← polarization of the cell surface into three distinct membrane compartments ← formation of a sealing zone ← resorption ← detachment ← cell death (apoptosis)
Cytoplasm
Cytoplasm packed with rough endoplasmic reticulum, stains blue with hematoxylin
Cytoplasm packed with mitochondria, for energy production associated with bone deletion. Clear areas contain material for export from cell.
Nucleus
Eccentric nucleus with adjacent Golgi apparatus
Multinucleated giant cell with ruffled border isolating highly acidic environment for mineral dissolution, enzymatic collagen removal, and abundant acid phosphatase
Survival
As blast, up to 10 days
As resident osteocyte, months to years
24 to 48 hours
Efficiency
Up to 100 osteoblasts required to fill bone deleted by one osteoclast; requires 3 months
Digests 3 times its cell volume in 24 hours
Clast 100 times more efficient than blast in terms of volume and creates its cavity in 1 day, while refill takes months
Clast efficiency is responsible for symptoms related to acute overuse (stress reactions leading to stress fractures)
After development of ossification centers:
Emergence of sequential primary and then secondary ossification centers subdivides long bone cartilage models (see Fig. 14-6).
Epiphysis (secondary ossification center)
Epiphyseal growth plates (physeal growth center/ physis)
Metaphysis
Diaphysis
Emergence of secondary ossification centers allows radiographic identification, providing a basis for evaluating chronological versus biologic bone age.
Primary Center of Ossification
The cartilage model in long bones begins as a central condensation of mesenchymal cells, which then produce cartilage matrix from the center of the bone, spreading outward toward the ends. This step, differentiation of cells to become chondrocytes, is regulated by a DNA transcription factor, Sox9, which controls the expression of genes coding for collagen (col), proteoglycan (PG), or non-collagenous proteins. Sox9, in conjunction with L-Sox5 and Sox6, binds to specific enhancer regions in the promoters of these target genes to activate gene transcription. Individual cartilage cells undergo sequential stages of development, which lead to development of the primary center of ossification.
Stages of Individual Cartilage Cell Development (Chon-drification)
Differentiation from uncommitted stromal cell
Rest
Reproduction
Maturation/matrix production
Hypertrophy
Matrix mineralization: first identifies primary center of ossification on x-ray
Senescence and death (apoptosis)
Stages of Development in Primary Center of Ossification
Initiation of chondrification
Begins near the center
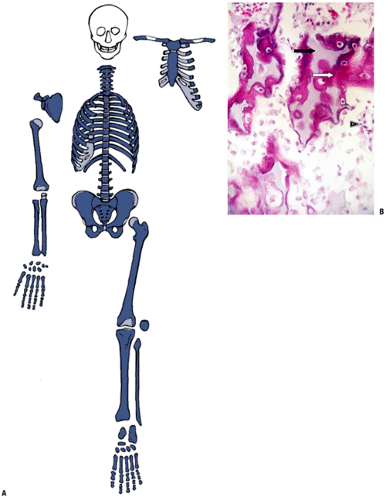
Cartilage cells there reach the final stages of mineralization and terminal differentiation first (Fig. 14-7).
Woven bone formation
Change in cartilage maturation induces the formation of a sleeve of coarsely woven bone at the level of maximum cartilage cell hypertrophy (see Fig. 14-7).
Stimulus for this induction is unknown.
Perichondrium changes to periosteum.
Vascular invasion
Death of cartilage cells may provide the signal for vascular invasion; it is likely that cytokines direct the process.
Invading vascular granulation tissue brings in endothelial cells and precursor cells that differentiate into blasts and clasts.
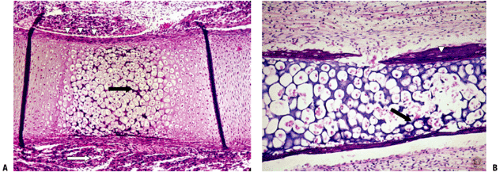
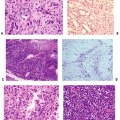
Figure 14-7 Primary center of ossification. (A) Central portion of cartilage model where cartilage cell death (absence of cells centrally) has left behind dark-staining calcified cartilage (black arrow), which will act as a nidus for apposition of woven bone in the formation of primary trabeculae; this defines enchondral bone formation. On either end, the cartilage cells undergo growth through their cell cycle, showing growth plate morphology. White arrow indicates surrounding mesenchymal anlage forming early skeletal muscle. (B) Later, vascular invasion occurs through a gap in the woven bone that has formed from the inner layer of the periosteum. The cortex at this time is a coarsely woven sleeve of bone, incomplete where the vessel enters. Cartilage cells are dead; the calcified cartilage black arrow) is removed by osteoclasts emerging as precursors from the vessels; no bone is deposited yet on the cartilage cores. Arrowheads indicate primitive cortex, arising from the cambium layer of the periosteum, which is woven bone in the embryo, lamellar bone in the child. (Courtesy of the Armed Forces Institute of Pathology, Washington, DC.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access