GONADOTROPIN-DEPENDENT/CENTRAL PRECOCIOUS PUBERTY
Gonadotropin-dependent PP (Table 92-1) results from premature activation of the hypothalamic–pituitary–gonadal axis. Girls may present with breast development alone or in combination with other secondary sexual development and menstrual bleeding. Since activation of the gonads, or gonadarche, is independent of adrenarche, pubic and axillary hair development may be less advanced than other secondary characteristics. Boys present first with testicular enlargement followed by phallic enlargement, pubic and axillary hair, body odor, and acne. Characteristic of this form of puberty, both girls and boys demonstrate accelerated growth velocities for age and advanced bone ages.
The etiology of gonadotropin-dependent PP is idiopathic in the vast majority of girls but in less than one-third of boys.17,18 The reason for this is unclear. It has been postulated to be due to the relative ease with which reactivation of the hypothalamic–pituitary–gonadal axis occurs in girls as compared to boys.
Idiopathic PP has also been reported with increasing frequency in girls adopted from developing countries.19 A correlation between the onset of early puberty and the rate of catch-up growth after adoption has been found, but the trigger of PP remains unknown. Final height may be compromised, worsening preexisting psychosocial adjustment problems.
Hypothalamic hamartomas are the most common identifiable causes of gonadotropin-dependent PP, being found in 10% to 44% of patients.18 Hypothalamic hamartomas are congenital malformations made up of heterotopic, nonneoplastic CNS tissue found attached to the hypothalamus between the tuber cinereum and the mammillary bodies, just posterior to the optic chiasm. Magnetic resonance imaging (MRI) scanning can identify hamartomas initially missed on computed tomographic (CT) scanning alone. Hypothalamic hamartomas are comprised mostly of ectopic GnRH neurons that may act as autonomous GnRH-pulse generators. These neurons are not regulated by the normal CNS mechanisms that inhibit hypothalamic GnRH neuron activity during the prepubertal years. As a result, synchronous pulsatile firing often begins before the normal age of puberty, usually before 4 years.18,20 Hypothalamic hamartomas can also be associated with gelastic (laughing) seizures, secondary generalized epilepsy, behavior problems, and variable cognitive degeneration.20 Hypothalamic hamartomas usually do not enlarge with time and rarely cause mass effects. Because GnRH-analog therapy is extremely effective,20,21 surgical resection is not recommended. Furthermore, long-term follow-up studies reveal that even if the hamartoma is completely resected, reversal of puberty is not always complete and pubertal advancement can resume prematurely.21
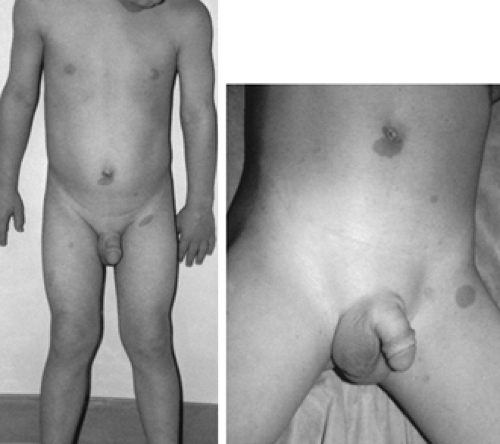
Many other CNS abnormalities can result in gonadotropin-dependent PP, most likely via disruption of tonic inhibitory input to the hypothalamus. Optic pathway gliomas are found frequently in association with neurofibromatosis type 1, but PP occurs only if the optic chiasm is involved22 (Fig. 92-1 and Fig. 92-2). Conversely, patients with the syndrome of septooptic dysplasia, myelomeningocele, or isolated hydrocephalus also have an increased incidence of PP.
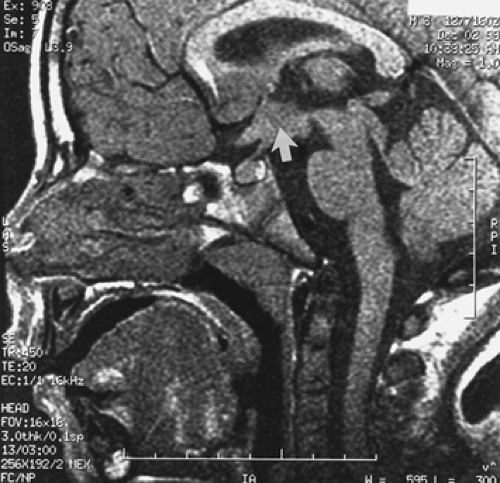 FIGURE 92-2. Head magnetic resonance imaging scan from the boy in Figure 92-1. Note the presence of a large glioma involving the optic chiasm (arrow).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|