HORMONE SECRETION
Part of “CHAPTER 51 – PARATHYROID HORMONE“
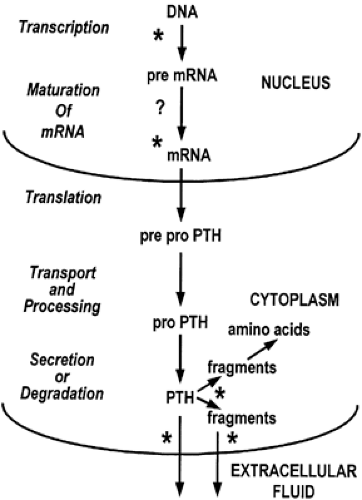
GENERAL FEATURES OF HORMONAL SECRETION
Relatively little PTH is stored in secretory granules within the parathyroid glands. In the absence of a stimulus for release, intraglandular metabolism occurs, causing complete degradation of the hormone to its constituent amino acids or partial degradation to fragments through a calcium-regulated enzymatic mechanism.32 In cases of hypercalcemia, the predominant hormonal entities released from parathyroid glands appear to be fragments composed of midregion or COOH-terminal sequences or both, containing little or no bioactivity.33,34 In cases of hypocalcemia, degradation of PTH within the parathyroid cell is minimized, and the major hormonal entity released appears similar to or identical with the bioactive PTH 1–84 molecule.35 Consequently, in the presence of hypocalcemia, increased amounts of bioactive PTH are secreted, even in the absence of additional synthesis of hormone. With a brief hypocalcemic stimulus, a biphasic secretory response often occurs. Hormone, presumably newly synthesized and derived from “immature” Golgi vesicles, is initially released in a large burst over a few minutes.2 This is followed by a lower response sustained for a longer period, presumably representing hormone stored in secretory granules. However, hormone stores are insufficient to maintain secretion for more than a few hours in the presence of a sustained severe hypocalcemic stimulus; although other mechanisms—transcriptional and posttranscriptional—increase PTH synthesis to some extent, additional PTH secretion ultimately depends on an increase in the number of parathyroid cells. Such an increase appears to be modulated by the reductions in circulating 1,25-dihydroxyvitamin D that often accompany hypocalcemia.
A second protein, identical to chromogranin A from the adrenal medulla, is cosecreted with PTH in most conditions leading to the release of PTH.36,37 This 50-kilodalton (kDa) protein is synthesized within the parathyroid gland and stored with PTH within secretory granules. This molecule, which can be glycosylated and phosphorylated, is a member of the chromogranin-secretogranin (granin) family of proteins that occurs in virtually all neuroendocrine cells.38,39 and 40 The family of proteins plays several roles in the process of regulated secretion, including targeting peptide hormones and neurotransmitters to secretory granules of the regulated secretory pathway. Chromogranin A also functions as a precursor of biologically active peptides that modulate neuroendocrine cell secretion in an autocrine or paracrine fashion (see Chap. 175).
MODULATORS OF PARATHYROID GLAND SECRETION
The calcium ion is the main regulator of parathyroid gland activity, although several other agents influence the release of PTH from parathyroid glands. These include various ions, agents altering the activity of the parathyroid cell adenylate cyclase system (e.g., β-adrenergic catecholamines, histamine), peptides derived from chromogranin A, and vitamin D metabolites.
A circadian rhythm has been reported for PTH secretion, with increased blood levels occurring at night and small amplitude pulses of PTH secretion occurring at much shorter intervals.41,42 These studies may suggest neural or central nervous system influences on PTH secretion, or they could reflect circadian alterations in the levels of extracellular fluid calcium.
IONS
Cations.
The most potent of the cations modulating PTH release and the secretagogue that is most important in altering PTH release under physiologic and pathophysiologic circumstances is the calcium ion (Fig. 51-3). Although there is an inverse relationship between ambient calcium levels and PTH release, this is a curvilinear rather than proportional relationship.43 From in vivo studies of cattle, maximal rates of PTH secretion of ˜16 ng/kg per minute appear to be rapidly achieved at calcium levels below 7.5–8.0 mg/dL (1.88–2.00 mmol/L). When calcium levels are reduced from 10.0 to 9.5 mg/dL (2.50–2.38 mmol/L), a small and gradual increase in PTH secretion occurs that does exhibit a proportional relationship to the ambient calcium level. Half-maximal secretion rates normally occur at calcium levels of ˜8.5 mg/dL (2.12 mmol/L), which is the set-point for PTH secretion. Basal secretion rates result after ambient calcium levels have risen above 11 mg/dL
(2.75 mmol/L) and appear to persist despite further increases in calcium concentration, even up to 16–18 mg/dL (4.00–4.50 mmol/L). Similar results have been observed in studies with human parathyroid tissue in vitro. The nonsuppressible (more correctly, non–calcium suppressible) component of constitutive PTH secretion appears to comprise mainly bioinactive midregion and COOH-terminal fragments. However, direct determination of the activity of hormonal material released when calcium levels are markedly elevated also demonstrates some bioactivity.
(2.75 mmol/L) and appear to persist despite further increases in calcium concentration, even up to 16–18 mg/dL (4.00–4.50 mmol/L). Similar results have been observed in studies with human parathyroid tissue in vitro. The nonsuppressible (more correctly, non–calcium suppressible) component of constitutive PTH secretion appears to comprise mainly bioinactive midregion and COOH-terminal fragments. However, direct determination of the activity of hormonal material released when calcium levels are markedly elevated also demonstrates some bioactivity.
This inverse relationship between PTH and extracellular calcium contrasts with the influence of the calcium ion as a secre-tagogue in most other secretory systems in which elevations in this ion enhance release of the secretory product. This distinction between the parathyroid cell and other secretory cells is maintained intracellularly, where elevations rather than decreases in cytosol calcium correlate with decreased PTH release.44 Alterations in extracellular fluid calcium levels are transmitted through a parathyroid plasma membrane calcium sensing receptor that couples through a G-protein complex to phospholipase C. Increases in extracellular calcium lead to increases in inositol 1,4,5-trisphosphate (IP3) and mobilization of intracellular calcium stores. The manner in which this inhibits hormone secretion is not understood. Although it would be anticipated that increases in diacylglycerol would accompany IP3 increases caused by hydrolysis of phosphoinositides, activate protein kinase C, and result in reduced PTH secretion, this does not appear to occur in the parathyroid cell.45 Paradoxically, agents that do stimulate protein kinase C, such as phorbol esters, stimulate rather than inhibit hormone secretion. This may be a result of phosphorylation of amino acids in the COOH-terminus of the CaSR, leading to its desensitization by blocking interaction of the receptor with its G protein. The precise steps in the pathway from changes in extracellular calcium levels to hormone release remain to be elucidated.
The parathyroid cell Ca2+-sensing receptor cDNA was identified by expression cloning in Xenopus laevis oocytes.46 The mRNA of the human receptor encodes a polypeptide of 1078 amino acids that is predicted to contain a very large extracellular domain of ˜600 amino acids and a seven transmembrane-spanning region characteristic of G-protein–coupled cell-surface receptors. Compared with the other known G-protein–coupled receptor family members, the Ca2+-sensing receptor shows some homology with the metabotropic glutamate, γ-aminobutyric acid-B, and vomeronasal odorant receptors, sharing conserved cysteine residues and a hydrophobic sequence in the NH2-terminal region. The Ca2+-sensing receptor has a low affinity for Ca2+, and consistent with this, the receptor sequence does not contain any of the Ca2+ binding motifs found in high-affinity calcium-binding proteins. Highly acidic regions in the extracellular NH2-terminal domain and the second extracellular loop may bind calcium as they do in other known low-affinity Ca2+ binding proteins. Besides the parathyroid, the Ca2+-sensing receptor is also expressed in other cells having Ca2+-sensing functions, such as those of the kidney tubule, the calcitonin-secreting thyroid C-cells; and in diverse other organs and tissues such as brain, keratinocytes, gastrointestinal tract, and retina. Neomycin binds the receptor, possibly accounting for the toxic renal effects of aminoglycoside antibiotics.
The human Ca2+-sensing receptor gene has been partially characterized and shown to consist of seven exons spanning more than 20 kb of genomic DNA.47 The long extracellular domain is encoded by the first two to six exons, and the remainder of the molecule is encoded by exon seven. Exons 1A and 1B encode two alternative 5′ untranslated regions of the CaSR mRNA. Inherited abnormalities of the CaSR gene, located on chromosome 3p13.3-21, can lead either to hypercalcemia or to hypocalcemia depending on whether they are inactivating or activating, respectively. Heterozygous loss-of-function mutations give rise to familial (benign) hypocalciuric hypercalcemia (FHH), in which the lifelong hypercalcemia is asymptomatic.47,48 and 49 The homozygous condition manifests itself as neonatal severe hyperparathyroidism (NSHPT), which is a rare disorder characterized by extreme hypercalcemia and the bony changes of hyperparathyroidism that occur in infancy.50,51 In several cases of NSHPT the parents are normocalcemic, and the cases seem to be sporadic. Autosomal-dominant hypocalcemia (ADH) is caused by gain-of-function mutations in the CaSR gene.52 Autosomal-dominant hypocalcemia may be asymptomatic or present with neonatal or childhood seizures. Because of the overactive CaSR in the nephron, these patients are at greater risk of developing renal complications during vitamin D therapy than are patients with idiopathic hypoparathyroidism. A common polymorphism in the intracellular tail of the CaSR, Ala to Ser at position 986, has a modest effect on the serum calcium concentration in healthy individuals.53 CaSR polymorphisms might also affect urinary calcium excretion and bone mass; therefore, the CaSR is a candidate gene for involvement in disorders such as idiopathic hypercalciuria and osteoporosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree