Genetic Basis of Kidney Cancer
W. Marston Linehan
Ramaprasad Srinivasan
Laura S. Schmidt
BACKGROUND
Kidney cancer affects over 57,000 annually in the United States and is responsible for nearly 13,000 deaths per year (1). Patients who are found to have localized renal tumors T1-T2 can have a 95% 5- or 10-year survival (2). However, those who present with metastatic disease have a 26% 5-year survival. Kidney cancer is not a single disease but is made up of a number of different types of cancer that occur in the kidney, each with a different histology, a different clinical course, and caused by alteration of different genes (3). Kidney tumors occur as clear cell (75%), type 1 or type 2 papillary renal carcinoma (15%), chromophobe renal carcinoma (5%), or other rare subtypes such as collecting duct, medullary renal carcinoma and oncocytoma (5%) (Fig. 41.1).
HEREDITARY FORMS OF KIDNEY CANCER
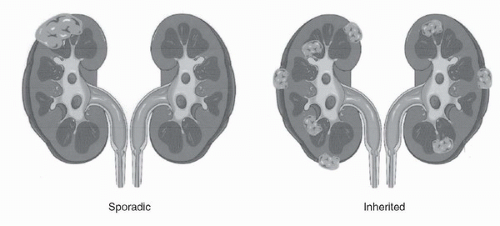
Kidney cancer, like prostate cancer, breast cancer, and colon cancer, occurs in both a familial (inherited) as well as a nonfamilial (sporadic) form. There are six types of inherited epithelial kidney cancer described so far: von Hippel-Lindau (VHL) (clear cell renal carcinoma), hereditary papillary renal carcinoma (HPRC) (type 1 papillary renal carcinoma), the Birt Hogg Dubé (BHD) syndrome (chromophobe renal carcinoma and oncocytoma), hereditary leiomyomatosis and renal cell carcinoma (HLRCC) (type 2 papillary renal carcinoma, and others), and those associated with alterations in succinate dehydrogenase (SDH) or tuberous sclerosis complex (TSC) (Fig. 41.2; Table 41.1).
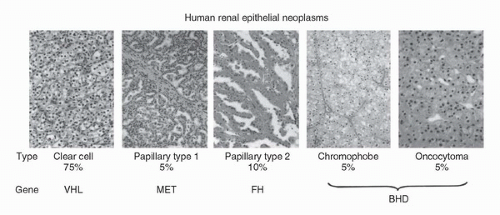 FIGURE 41.1. Kidney cancer is made up of a number of different types of cancer, each with a different histology, a different course, responding differently to therapy, and caused by alteration of a different gene. (Reproduced from Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol 2003;170(6 Pt 1):2163-2172, with permission.) (See color insert.) |
Von Hippel Lindau: Clear Cell Renal Carcinoma
VHL is an inherited form of renal carcinoma in which affected individuals have a predisposition for the development of tumors in a number of organs, including the kidneys. VHL patients are at risk for the development of early onset, bilateral, multifocal renal tumors and cysts. The renal tumors are uniformly of clear cell type. It has been estimated that these patients may develop up to 600 tumors and 1100 cysts per kidney. These tumors tend to occur early in life, sometimes as early as the second decade. VHL-associated renal carcinomas can metastasize if not detected and treated in a timely fashion. Historically, 35% to 45% of VHL patients died of renal carcinoma. The surgical management of renal carcinoma in VHL patients is discussed in Bratslavsky et al., Chapter 44G. Because of the multifocal nature of VHL-associated renal carcinoma and the need for repeat surgery for recurrent lesions, the current recommendation is for management of small renal tumors with observation until the largest tumor reaches 3 cm. Nephron-sparing surgery is then recommended (Fig. 41.3) (Table 41.2).
VHL patients are also at risk for the development of pheochromocytomas. These may occur in children before the age of 10 and may be bilateral, multifocal, and extraadrenal (4,5). These patients may also develop pancreatic islet cell tumors and cysts. The islet cell tumors are malignant and can metastasize (6). Other manifestations of VHL include CNS and spinal hemangioblastomas, endolymphatic sac tumors, and retinal angioma. Neither CNS hemangioblastoma nor retinal angiomas are malignant; however, both can be associated with significant morbidity.
TABLE 41.1 INHERITED FORMS OF RENAL CARCINOMA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TABLE 41.2 CLINICAL FEATURES OF VHL | |||||||
|---|---|---|---|---|---|---|---|
|
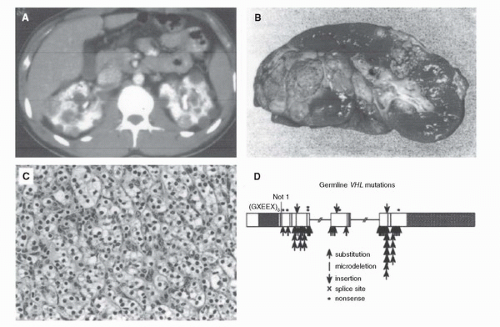 FIGURE 41.3. VHL-associated renal carcinoma may be bilateral and multifocal (A,B) clear cell kidney cancer (C) and may appear as early as the second decade. Affected individuals in VHL kindreds are characterized by germline mutation of the VHL gene (7). (Reproduced from Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol 2003;170(6 Pt 1):2163-2172, with permission.) (See color insert.) |
Identification of the VHL Gene
In order to identify the VHL gene, investigators conducted genetic linkage analysis in VHL families. The VHL gene was identified on the short arm of chromosome 3 (7). The VHL gene was found to be mutated in the germline of patients affected by VHL (8). This provided a clinical test to make the diagnosis of VHL in at-risk individuals in VHL kindreds.
VHL: Clear Cell Kidney Cancer Gene
When tumor tissue from patients with sporadic (noninherited) kidney cancer was evaluated for alteration of the VHL gene, mutation of this gene was found in a high percentage of tumors from patients with clear cell renal carcinoma (9,10). VHL gene mutations were not found in tumors from patients with papillary renal carcinoma, chromophobe renal carcinoma, or oncocytoma. The VHL gene is the gene for the common form of sporadic clear cell renal carcinoma (Fig. 41.4).
Clinical Implications
Understanding the genetic basis of cancer of the kidney provides a method for molecular classification of the cancer and could provide enhanced methods for diagnosis of this disease. Yao et al. have shown that there is an improved survival rate in patients with sporadic clear cell renal carcinoma with VHL mutation (11). VHL mutations have been detected in circulating cells, lymph nodes, and urine of patients with clear cell renal carcinoma, providing possible approaches for early diagnosis of this disease. Most importantly, understanding the VHL gene pathway has provided the foundation for the development of targeted therapeutic approaches to patients with advanced, clear cell kidney cancer.
 FIGURE 41.4. VHL gene: clear cell renal carcinoma (A). VHL gene mutations have been found in a high percentage of tumors from patients with sporadic, nonhereditary clear cell renal carcinoma (9,10) (B). VHL gene mutations have not been found in type 1 or type 2 papillary renal carcinoma, chromophobe renal carcinoma, or oncocytoma. (Reproduced from Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 1994;7(1):85-90, with permission.) (See color insert (A).) |
VHL Gene Pathway: the VHL Complex Targets Hypoxia-Inducible Factors for Degradation
The VHL protein forms a multiprotein complex with elongins c and b, and Cul2 to target the alpha subunits of the hypoxia-inducible factors (HIFs), HIF1α and HIF2α for ubiquitin-mediated degradation (12,13,14,15,16). This is an oxygendependent process. In normoxia, the VHL complex targets HIF and promotes its degradation. During hypoxia, the complex cannot degrade HIF α subunits and HIF overaccumulates in the cell. HIF is a transcription factor that regulates the transcription of a number of genes important in cancer, such as vascular endothelial growth factor (VEGF), transforming growth factor alpha (TGFα), platelet-derived growth factor (PDGF), and epidermal growth factor receptor (EGFR) (17,18) (Fig. 41.5).
VHL Gene Pathway: Opportunities for Disease-Specific Therapy for Clear Cell RCC
One approach to the development of effective forms of therapy for patients with advanced clear cell renal carcinoma is to develop agents which target the VHL pathway. To date, the Food and Drug Administration (FDA) has approved six therapeutic agents that target the VHL pathway in patients with advanced clear cell kidney cancer. The first agent used to target the VHL pathway in patients with advanced clear cell kidney cancer was bevacizumab, a monoclonal antibody which targets VEGF (19). Sorafenib and sunitinib target the vascular endothelial growth factor receptor (VEGFR) and the platelet-derived growth factor receptor (PDGFR), everolimus and temsirolimus target the mammalian target of rapamycin (mTOR) and are thought to affect HIF translation, and pazopanib targets the VEGFR1/R2/R3 receptors (20,21,22,23,24). In a randomized phase III study in previously untreated patients with metastatic clear cell RCC, sunitinib has been reported to induce RECIST partial responses in 31% of patients with an improvement in both progression-free survival and overall survival compared to interferon α (21); however, there are few complete responses and most patients eventually progress. Further studies are in progress to identify more effective forms of therapy targeting the VHL kidney cancer gene pathway for the treatment of patients with advanced clear cell kidney cancer (Fig. 41.6).
Hereditary Papillary Renal Carcinoma: Type 1 Papillary Renal Carcinoma
HPRC is a hereditary cancer syndrome in which affected individuals are at risk for the development of bilateral, multifocal papillary renal carcinoma. HPRC is inherited in an autosomal, dominant fashion. HPRC patients are at risk for developing bilateral, multifocal type 1 papillary renal carcinoma (25). It has been estimated that these patients may develop up to 3,000 tumors per kidney (26).
In order to identify the gene for the hereditary form of type 1 papillary renal carcinoma, genetic linkage analysis was performed in HPRC families. The protooncogene, Met, was found to be the gene for HPRC (27). Met is a protooncogene; it is the cell surface receptor for the ligand, hepatocyte growth factor (HGF). Activating mutations of Met were
identified in the germline of affected HPRC patients (27,28) and have been found in tumors in a subset of patients with noninherited, sporadic type 1 papillary renal carcinoma (29) (Table 41.3).
identified in the germline of affected HPRC patients (27,28) and have been found in tumors in a subset of patients with noninherited, sporadic type 1 papillary renal carcinoma (29) (Table 41.3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree