In 2008, an estimated 184,450 new cases of breast cancer will occur in the United States.1 In the same year, it is estimated that almost 41,000 women will die of breast cancer. These numbers demonstrate the impact of breast cancer among American women, but they cannot begin to address the diversity and complexity of the treatment of this malignancy. As research and advances in the care of the breast cancer patient occur, the need for greater understanding of the tenets and principles of clinical decision-making in the treatment of breast cancer increases.
In 1995, the National Comprehensive Cancer Network (NCCN) was developed in order to respond to the challenges of treating the most common cancers through clinical practice guidelines that delineated treatment through nonbiased, methodical algorithms. Based upon the Institute of Medicine’s definition, which described guidelines as “systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances,” the NCCN established a guideline program that proceeds as follows: comprehensive literature review by NCCN staff, development of prototype guidelines, review by site-specific panels, further analysis and clarification of guidelines, assessment by each NCCN member institution, collation of institutional review suggestions and issues, appraisal of the complete version of the guidelines by the NCCN Guidelines Steering Committee, and final approval by the NCCN Board of Directors. The guidelines are subsequently reviewed annually by the panel chair and 3 other multidisciplinary members to determine if further revisions are necessary. This chapter discusses the guidelines set forth by the NCCN regarding the treatment of primary invasive breast cancer (Figs. 82-1, 82-2, 82-3 and 82-4).
Figure 82-1
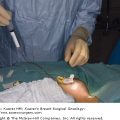
Algorithm of NCCN guidelines for the treatment of hormone receptor–positive, HER-2-positive breast cancer. [Reproduced with permission from The NCCN (2.2008) Invasive Breast Cancer. Clinical Practice Guidelines in Oncology. © National Comprehensive Cancer Network, 2008. Available at: http://www.nccn.org. To view the most recent and complete version of the guideline, go online to www.nccn.org.]
Figure 82-2
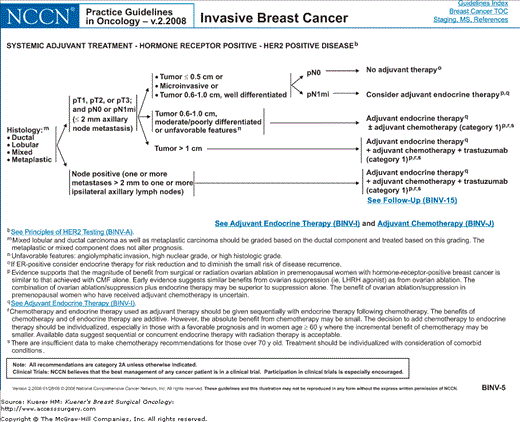
Algorithm of NCCN guidelines for the treatment of hormone receptor–positive, HER-2-negative breast cancer. [Reproduced with permission from The NCCN (2.2008) Invasive Breast Cancer. Clinical Practice Guidelines in Oncology. © National Comprehensive Cancer Network, 2008. Available at: http://www.nccn.org. To view the most recent and complete version of the guideline, go online to www.nccn.org.]
Figure 82-3
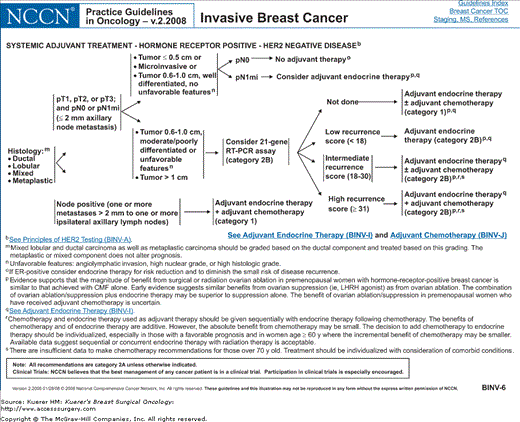
Algorithm of NCCN guidelines for the treatment of hormone receptor–negative, HER-2-positive breast cancer. [Reproduced with permission from The NCCN (2.2008) Invasive Breast Cancer. Clinical Practice Guidelines in Oncology. © National Comprehensive Cancer Network, 2008. Available at: http://www.nccn.org. To view the most recent and complete version of the guideline, go online to www.nccn.org.]
Figure 82-4
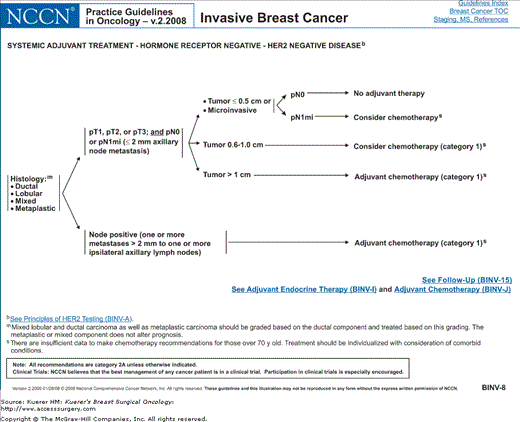
Algorithm of NCCN guidelines for the treatment of hormone receptor–negative, HER-2-negative breast cancer. [Reproduced with permission from The NCCN (2.2008) Invasive Breast Cancer. Clinical Practice Guidelines in Oncology. © National Comprehensive Cancer Network, 2008. Available at: http://www.nccn.org. To view the most recent and complete version of the guideline, go online to www.nccn.org.]
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) has demonstrated that the use of an anthracycline-containing chemotherapy and 5 years of tamoxifen may decrease mortality from breast cancer by more than 50% in women under 50 years of age, and by slightly less than 50% in women aged 50 to 69 years.2 Thus, for patients under the age of 70 years, adjuvant therapy demonstrates significant benefit. To assist in risk stratification for this diverse population, Adjuvant! Online, a prospectively validated Web-based tool, may be utilized to estimate the benefit of adjuvant chemotherapy and endocrine therapy upon 10-year disease-free survival (DFS) and overall survival (OS) in patients with HER-2-negative breast cancer.3,4 This program incorporates the patient’s age, comorbidities, tumor size, tumor grade, number of positive lymph nodes, and estrogen receptor (ER) status in order to calculate these values.
The NSABP B-18 trial demonstrated that the administration of chemotherapy in the neoadjuvant setting did not improve DFS or OS when compared to adjuvant administration.5 However, the use of neoadjuvant chemotherapy may offer certain advantages. Specifically, patients with pathologic complete response (pCR) following neoadjuvant chemotherapy have been found to have improved DFS and OS,6,7 and neoadjuvant chemotherapy is associated with a higher rate of successful breast-conserving therapy than adjuvant therapy.5 However, given the equivalency of benefit in the neoadjuvant and adjuvant settings, the chemotherapeutic regimens that are recommended in the adjuvant setting are appropriate for consideration in the neoadjuvant setting.
The EBCTCG’s overview demonstrated that the use of polychemotherapy significantly reduced the risk of recurrence and mortality at 15 years.2 Comparison of CMF (cyclophosphamide, methotrexate, and 5-fluorouracil) with anthracycline-containing regimens demonstrated that the use of anthracycline-based therapies resulted in a breast cancer mortality rate ratio of 0.84 (p < 0.00001) when compared to CMF. Thus, the NCCN recommends that anthracycline-containing therapies be the preferred regimen for node-positive patients. However, the EBCTCG did not analyze the effect of HER-2 status upon the efficacy of anthracycline-based treatment. Retrospective evaluation has shown that the benefit of anthracycline-based chemotherapy may be closely linked to its effect on HER-2-positive tumors.8-12 In addition, several trials have found that anthracycline-based therapies may have greater benefit in patients with HER-2-positive breast cancer, leading the NCCN to note this relationship in its guidelines.11-14
Randomized trials comparing AC (doxorubicin and cyclophosphamide) to CMF have shown no significant differences in relapse-free and overall survival.15-17 However, 2 trials involving node-positive patients that randomized patients to CEF (cyclophosphamide, epirubicin, and 5-fluorouracil) versus CMF (cyclophosphamide, methotrexate, and 5-fluorouracil) demonstrated that the use of CEF resulted in a statistically significant improvement in relapse-free survival, with one trial demonstrating a significant increase in overall survival.18,19
The addition of a sequential taxane-based regimen became accepted after the NSABP B-28 and the Cancer and Leukemia Group B (CALGB) Intergroup trials demonstrated that the sequential addition of paclitaxel to AC (Adriamycin [doxorubicin] and Cytoxan [cyclophosphamide]) resulted in a significant improvement in DFS, with the CALGB Intergroup trial showing that the addition of sequential paclitaxel therapy to AC resulted in an absolute benefit of 3% in overall survival at 5 years.20,21 Retrospective analysis of the CALGB Intergroup study demonstrated that the absolute benefits in DFS and OS due to chemotherapy were greater for patients with ER-negative, compared with ER-positive, tumors.22
Given the efficacy of taxanes, as well as their lack of cross-resistance with anthracyclines, a multicenter, randomized trial compared the effect of adjuvant TAC (docetaxel, doxorubicin, and cyclophosphamide) with FAC (5-fluorouracil, doxorubicin, and cyclophosphamide) upon DFS in patients with node-positive breast cancer.23 Investigators found that TAC resulted in an improvement in DFS at 5 years (75% vs 68%, p = 0.001). Treatment with TAC, in comparison to FAC, was associated with a 30% lower risk of death (HR 0.70, p = 0.008). However, the incidence of grade 3 or greater nonhematologic adverse events, grade 3 or greater neutropenia, and grade 3 or greater infections was higher in the TAC group.
To determine the optimal choice and schedule of taxane, the Eastern Cooperative Oncology Group E1199 randomized patients to treatment with paclitaxel or docetaxel given at 3-week intervals for 4 cycles or 1-week intervals for 12 doses; taxane therapy followed 4 cycles of AC therapy.24 The trial found that, when compared with patients receiving paclitaxel every 3 weeks, the odds ratio for DFS was 1.27 among those receiving weekly paclitaxel (p = 0.006), 1.23 among those receiving docetaxel every 3 weeks (p = 0.02), and 1.09 among those receiving weekly docetaxel (p = 0.29). The weekly administration of paclitaxel also resulted in an improved overall survival (odds ratio, 1.32; p = 0.01) over the 3-week interval regimen.
Taxane-based regimens were further studied in a phase III trial, which randomized patients with operable breast cancer to adjuvant therapy with either AC (doxorubicin and cyclophosphamide) or TC (docetaxel and cyclophosphamide).25 At 5 years, TC was associated with a significant improvement in DFS rate (HR 0.67, p = 0.015), with a trend toward an improvement in OS (HR 0.76, p = 0.13). These findings support consideration of TC as a regimen in the treatment of early breast cancer, but further studies are necessary to evaluate whether the exclusion of anthracyclines altogether is warranted in this setting.
Dose density has been evaluated in 5 trials; only 2 of these studies have demonstrated a benefit to the reduction in interval between treatments.26-30 Of these trials, the only trial to include taxanes and to evaluate the benefit of dose density in the absence of dose alterations was the CALGB Intergroup trial 9741, which compared the effects of concurrent versus sequential chemotherapy (doxorubicin followed by paclitaxel followed by cyclophosphamide, vs doxorubicin plus cyclophosphamide followed by paclitaxel) given either every 2 weeks (with filgrastim support) versus every 3 weeks.27 While there was no difference between the concurrent and sequential scheduling of these regimens, dose-dense therapy resulted in a 26% improvement in DFS (p = 0.01) and a 31% improvement in OS (p = 0.013).
The real first generation of targeted therapy was endocrine therapy, blocking the binding of the estrogen and/or progesterone receptors (REF). Targeting of the estrogen and/or progesterone receptors enabled clinicians to treat patients with therapies that demonstrated both significant efficacy and decreased toxicity, in comparison to traditional cytotoxic regimens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree