INTRODUCTION
SUMMARY
Lymphomas are a heterogeneous group of malignancies that originate from neoplastic transformation of lymphocytes that have undergone mutations that confer growth and survival advantages compared to their normal cellular counterparts.* These neoplasms usually originate in lymph nodes or lymphatic tissue in other sites (extranodal lymphoma), and can be localized or widespread at the time of diagnosis. Men are affected more frequently than women and the risk of acquisition of most lymphomas increases logarithmically with age. Classification systems have considered the likely lymphoid progenitor that corresponds to the phenotype (immunotype) and genotype of the malignant cells in the transformed clone. The specific pathologic diagnosis is usually established by the appearance of the histopathology in tissue sections, the immunophenotypic profile of CD antigens expressed on affected lymphocytes, specific cytogenetic findings, especially translocations (e.g., t[11;14]), immunocytochemical markers (e.g., cyclin D1), and the specific tissue location (e.g., mucosa-associated lymphatic tissue). Although most lymphomas arise without an evident cause, human T-cell leukemia/lymphoma virus I (HTLV-1), Epstein-Barr virus, hepatitis C virus, and human herpes virus-8 infections, as well as infections with the bacteria Helicobacter pylori and, perhaps, Chlamydophila psittaci, either are established as causal (e.g., HTLV-1) or have very strong associations with lymphoma incidence (hepatitis C virus), suggesting their role in causation. HIV is permissive by inducing severe immunodeficiency and setting the stage for an Epstein-Barr virus–induced or human herpes virus-8–induced lymphoma. These relationships may vary by geographic area. Several occupational and industrial exposures are suspected of being related to lymphoma incidence, for example, organochlorines, phenoxyacid herbicides, and others, but these associations have not been established with scientific certainty. At present, the estimated attributable risk of lymphoma from all suspected exogenous factors together is relatively small in proportion to the number of annual cases, leaving most cases without an apparent cause. There are wide discrepancies in the incidence of specific lymphoma subtypes in different geographic regions (e.g., follicular lymphoma is very common in the United States and very uncommon in East Asia). Primary extranodal lymphoma may involve virtually any tissue or organ. Depending on the site, important functional abnormalities may ensue (e.g., bilateral adrenal gland replacement and hypoadrenocorticism, hypothalamic–pituitary involvement and diabetes insipidus). Multidrug chemotherapy combinations in conjunction with lymphocyte-specific monoclonal antibody therapy form the foundation of current treatment paradigms for most lymphomas, though radiotherapy and surgical excision continue to play limited roles in selected circumstances, depending on the site and histopathology.
Acronyms and Abbreviations
ALCL, anaplastic large cell lymphoma; ALK, ALCL tyrosine kinase gene; ATLL, adult T-cell leukemia/lymphoma; CBC, complete blood count; CRu, complete remission unconfirmed; CT, computed tomography; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein-Barr virus; FDG,2-fluorodeoxyglucose; HHV-8, human herpesvirus-8; HL, Hodgkin lymphoma; HTLV-1, human T-cell leukemia/lymphoma virus-1; iFISH, interphase fluorescence in situ hybridization; Ig, immunoglobulin; IWG, International Working Group; MALT, mucosa-associated lymphatic tissue; NCCN, National Cancer Center Network; NHL, non-Hodgkin lymphoma; NK, natural killer; PET, positron emission tomography; R-CHOP, rituximab-cyclophosphamide, hydroxydoxorubicin, vincristine (Oncovin), and prednisone; REAL, revised European-American classification of lymphoid neoplasm; RNA-seq, ribonucleic acid sequencing; SEER, Surveillance, Epidemiology, and End Results; TCE, trichloroethylene; TCR, T-cell receptor; WHO, World Health Organization.
*Kenneth A. Foon was a coauthor of this chapter for the 8th edition of Williams Hematology and significant portions of that chapter have been retained.
DEFINITION AND HISTORY
Lymphomas are a heterogeneous group of malignancies of B cells, T cells, and, rarely, natural killer (NK) cells that usually originate in the lymph nodes, but may affect any organ of the body. Lymphoma previously was referred to as lymphosarcoma and its two major subtypes designated reticulum cell sarcoma and giant follicular lymphoma (Brill-Symmers disease).1,2,3 In 1966, Rappaport4 published a classification system based on the patterns of lymphoma cell growth, size, and shape that attempted to correlate morphology with clinical outcome. The classification proved to have some inaccuracies, such as the term histiocytic lymphoma to describe lymphoid tumors of large transformed lymphocytes that were not derived from the monocyte-macrophage lineage. Nonetheless, the Rappaport classification was an important milestone and became the most widely used classification in the United States. In 1974, Lukes and Collins proposed another classification system, which incorporated morphology with immunologic subtype, that was endorsed by the Committee on Nomenclature.4 Another scheme, the Kiel classification, introduced by Karl Lennert and colleagues, had been more popular in Europe.5 By the 1970s at least six classifications of lymphoma had been published, and the major ones included two in the United States, one in continental Europe, and one in the United Kingdom. There was no success in reaching a consensus classification that could be used worldwide. A National Cancer Institute study showed that there was poor reproducibility among different pathologists looking at the same slides and trying to classify the case of lymphoma using any existing scheme. In 1982, a Working Formulation sponsored by the National Cancer Institute attempted to reconcile the large number of competing classifications then in use.6 The Working Formulation was clinically useful and gained wide popularity. It divided the specific subtypes among high-grade, intermediate-grade, and low-grade lymphomas, focusing in part on the expected rate of progression, and not just on the phenotype of the case in question. With advances in our understanding of the immune system and lymphocyte progenitor developmental sequences, and the availability of monoclonal antibodies for subtyping lymphoid cells and lymphocyte gene profiling, a new classification schema became possible based on cell type, tissue of origin, immunophenotype, and genotype.
In 1994, a revised European-American classification of lymphoid neoplasms (the REAL classification) was proposed by the International Lymphoma Study Group (Chaps. 90 and 96).7 This group distinguished three major categories of lymphoid malignancies, which included B-cell, T-cell, and Hodgkin lymphoma. Lymphomas were defined by morphologic, immunologic, and genetic techniques. Many of the lymphomas were associated with distinct clinical presentations, and cases that did not fit into defined entities were left unclassified. Further subclassification8 divided each of the B-cell and T-cell lineages into (1) indolent lymphomas (low risk of rapid progression), (2) aggressive lymphomas (intermediate risk of progression), and (3) very aggressive lymphomas (high risk of progression). In 1995, a collaborative project of the European Association for Haematopathology and the Society for Hematopathology began to revise the REAL classification. In 2001, they published the World Health Organization (WHO) Classification of Tumors of the Haematopoietic and Lymphoid Tissues that is used in this chapter and represents the current worldwide consensus classification of malignancies that arise in a lymphocyte. In 2008, the WHO updated this classification (Chaps. 90 and 96).9,10
Emerging concepts in the staging, therapy, and response evaluation of lymphomas evolved in parallel with the changing nomenclature. Initial efforts to define the extent of disease involvement were undertaken primarily for Hodgkin lymphoma (HL) and ultimately led to the Ann Arbor classification,11 which subdivided patients into four stages, each subclassified into A and B groups based on the presence of fevers greater than 38.3°C, weight loss, and drenching night sweats. The Ann Arbor system proved useful for decades and was subsequently applied also to non-Hodgkin lymphomas (NHLs). The Cotswold modification of the Ann Arbor Classification12 first formally incorporated computed tomography (CT) into clinical staging paradigms and introduced the terms “X” for bulky disease and “complete remission unconfirmed” (CRu) to describe patients with a residual mass after treatment that most likely represented fibrous scar tissue. The sensitivity and accuracy of CT imaging of the abdomen rendered the “staging laparotomy” for assessment of the abdomen obsolete. The first universally accepted response criteria for NHL were initially published in 1999 by the National Cancer Institute Working Group13 and later revised in 2007 to incorporate positron emission tomography (PET), marrow immunohistochemistry and flow cytometry for response assessment.14 PET/CT imaging rendered the CRu designation obsolete in 2007, because residual radiographic abnormalities could be accurately determined to be either residual active lymphoma or posttreatment fibrosis, based on the metabolic activity of the lesions. These criteria were critically reviewed and analyzed by working groups at the 11th and 12th International Conferences on Malignant Lymphoma in Lugano, Switzerland, in 2011 and 2013 and at the 4th International Workshops on Positron Emission Tomography in Lymphoma in Menton, France in 2012.15,16 These international workshops were attended by leading hematologists, oncologists, radiation oncologists, pathologists, radiologists, and nuclear medicine physicians, representing all major lymphoma clinical trials groups and cancer centers in North America, Europe, Japan, and Australasia. Their deliberations culminated in the publication in 2014 of improved criteria for the initial evaluation, staging and response assessment for both HL and NHL17 that are relevant for community physicians, investigator-led trials, cooperative groups, and registration trials. These new rules, known as the “Lugano Classification,” depart substantially from older staging and evaluation systems as detailed later in this chapter.
EPIDEMIOLOGY
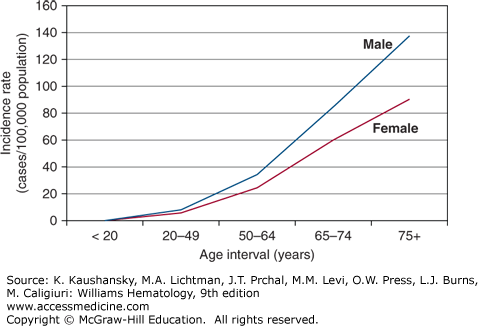
Approximately 79,990 new cases of lymphoma were projected to be diagnosed and approximately 20,170 persons in the United States were expected to die of this disease in 2014.18 These numbers represent 4.8 percent of the annual incidence of all cancers and 3.4 percent of annual cancer-related deaths. The most recent age-adjusted incidence rates per 100,000 population provided by Surveillance, Epidemiology, and End Results (SEER) Program of the United States National Cancer Institute are: 24.9 for white males, 17.4 for black males, 17.2 for white females, and 11.9 for black females.19 The increased risk for men is similar to that found in other countries, although the incidence of NHL in the United States is approximately threefold that of several underdeveloped countries and twofold that of several comparable industrialized countries.20 The risk of developing lymphoma is less in the United States among persons of African descent in comparison to those of European descent. An exponential increase in incidence in NHL among men and women occurs with increasing age (Fig. 95–1). There are some notable exceptions to this overall trend, however, with lymphoblastic lymphoma occurring most commonly in children, Burkitt lymphoma in the 20- to 64-year-old age group, and primary mediastinal B-cell lymphoma developing at a median age of 35 years.20
Figure 95–1.
The graph depicts the rate of increase with age in non-Hodgkin lymphoma incidence among American males and females. This pattern is true for Americans of European or of African descent. (Data from the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973-2011), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.; 2014.)
Follicular lymphoma represents approximately 25 percent of NHL cases in the United States, but is very uncommon in many developing countries and in Asia, especially in Japan and China.21,22 The United States has a higher incidence of all lymphomas than does Japan, whereas the incidence of extranodal lymphoma is higher in Japan.21,22 Burkitt lymphoma occurs most frequently in sub-Saharan Africa, whereas T-cell leukemia/lymphoma is most common in southwest Japan, the southeastern United States, northeastern South America, and the Caribbean basin.
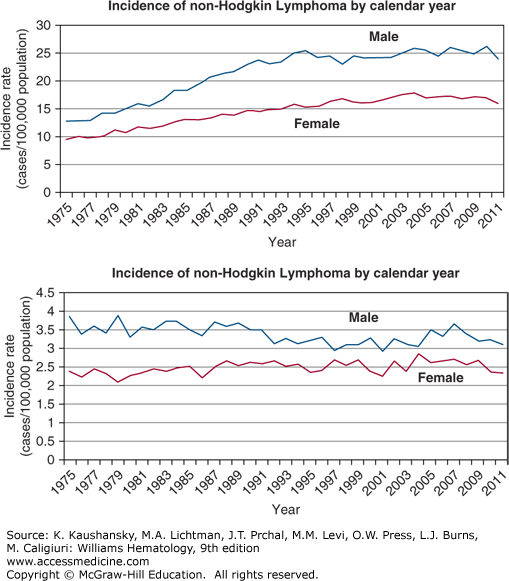
The incidence of NHL increased dramatically in the last half of the 20th century in Europe, Asia, and in the United States.20,21,22 From 1973 to 1990, inclusive, the increase in the United States was slightly more than 80 percent, or approximately 4 to 5 percent per year (Fig. 95–2). The increase in incidence started after World War II, but the best data in the United States were acquired after 1972. The increase affected men and women, all age groups except children, and most histologic types examined, though the greatest increase was in diffuse large B-cell lymphoma (DLBCL). The increased incidence per year reached a plateau in the early 1990s, except among women and older men where the incidence continued to rise.21 Hardell has hypothesized that new chemicals synthesized during and after World War II accounted for increased incidence trends, with subsequent improvements in safety gear and regulatory restrictions resulting in stabilization in the last decade.23 HIV was not prevalent in the human population when the increase first became apparent, although in later years HIV-related lymphoma may have played a part in increasing incidence rates. Orbital adnexal lymphoma and mantle cell lymphoma are exceptions to the recent plateau, with each still increasing at approximately 6 percent a year.24,25
Figure 95–2.
Incidence of non-Hodgkin and Hodgkin lymphoma by calendar year. The incidence of non-Hodgkin lymphoma approximately doubled from the early 1970s to the mid-1990s in the United States and in other industrialized countries that tracked incidence of specific cancers. No satisfactory explanation has been uncovered for this change. The “epidemic” of lymphoma ended in the mid-1990s and the incidence curves have been “flat” since 1996. The increase in incidence was present in Americans of European and African descent and among men and women. In stark contrast and serving as an internal control, the incidence of Hodgkin lymphoma is essentially unchanged over that period of time. (Data from the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973-2011), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.; 2014.)
Several occupations and industries and several potentially hazardous exposures, such as pesticides, herbicides, dyes, engine exhausts, and solvents, have been found in one study or another to be more frequent in lymphoma patients than “matched” healthy comparison groups.21,26,27 The results often have been inconsistent from study to study. Expert opinion indicates that no workplace exposure has been conclusively linked to lymphoma,28 although farming or living in a community in which farming is prevalent has been a frequent association with higher lymphoma incidence.21,26,27
Many publications have reported a slightly increased familial predisposition to the development of NHL, with an odds ratio of 1.5 in first-degree relatives of patients with NHL in a pooled analysis of 17 case control studies.20,29,30,31 Nonsyndromic familial lymphoma refers to apparently healthy family members, unlike syndromic familial lymphoma in which immunodeficiency syndromes are the predisposing phenotype (e.g., Wiskott-Aldrich syndrome; see “Immunosuppression” below). The familial cases occur in different generations and among enough family members to strongly suggest that a predisposing unidentified gene results in an incidence above that in the population at large. Li-Fraumeni syndrome is such an example, involving germline mutations in p53. Alternatively, it is possible that family members inherit a susceptibility to an unidentified environmental lymphomagen.
ETIOLOGY AND PATHOGENESIS
Unlike other cancers, the malignancy referred to as “lymphoma” consists of nearly 80 phenotypes.9 Because each subtype of lymphoma exhibits unique nuances in its natural history, therapeutic considerations, and prognosis, unequivocal establishment of the precise subtype of lymphoma is of paramount importance in a newly diagnosed patient. The single most important test in a lymphoma patient is an adequate biopsy of affected tissue. In most cases, this should be an excisional lymph node biopsy, or generous incisional biopsy of an extranodal site. When the only sites of disease involvement are deep in the thorax or pelvis, rendering excisional node biopsy difficult, a core needle biopsy obtained with the assistance of ultrasound or CT guidance may be adequate. Fine-needle aspiration alone should never be used as the sole method of establishing the initial diagnosis of lymphoma,17,32,33 because the precise subtyping of lymphomas requires examination of tissue architecture, not merely cytologic examination of isolated cells. Biopsies should be subjected to microscopic examination, flow cytometry of fresh cells, immunohistochemical examination of fixed tissue sections, and cytogenetic/interphase fluorescence in situ hybridization (iFISH) analysis.33 In the near future, it is likely that genomic analyses using ribonucleic acid sequencing (RNA-seq) technology will also become important to define specific mutations permitting personalized selection of targeted agents capable of inhibiting deranged intracellular pathways.34
Table 95–1 lists the most prevalent phenotypes distinguishable by pathologists according to the current WHO classification system.9,10 Approximately 88 percent of lymphomas originate in a cell that exhibits features most consistent with B lymphocyte derivation (B-cell CD surface antigens or immunoglobulin gene rearrangement). The remaining cases are lymphomas in which the phenotype and genotype are most closely related to T cells or NK cells (T-cell receptor [TCR] chain rearrangements or specific immunophenotypes). This heterogeneity is problematic for histopathologic diagnosis, classifying patients in clinical trials, and selecting therapy. It also makes studies of epidemiology and etiology more difficult. To understand acquired (environmental) or genetic causes of the type of lymphoma in question, the latter studies, to be insightful, must stratify the study group by specific histopathologic diagnosis. This requirement can be difficult if one is studying uncommon phenotypes.
|
The histopathologic diversity of lymphoma is the result of the complexity of the immune system; its wide distribution through many organs with highly specialized sites, such as mucosa-associated lymphatic tissue (MALT); its differentiation into T-lymphocyte, B-lymphocyte, and NK-lymphocytic lineages; its complex maturation through many progenitor cell levels; the concomitant sequential alterations in expression of immune complex genes and the myriad opportunities for transforming mutations; and the transforming effects of mutations in the many genes encoding immunoglobulin chains or the TCR. Thus, studies in prevalent subtypes of lymphoma such as follicular and DLBCL are more common than in uncommon or rare subtypes. In addition, epidemiologic or etiologic findings relevant to one subtype may not be relevant to another subtype or to lymphoma in general.
An increased incidence of lymphoma has been observed, especially among farmers and gardeners but also in printers, woodworkers, dry cleaners, barbers and hairdressers, possibly from organic solvent exposure, especially to trichloroethylene (TCE), a solvent used in many industries.20,21,22,26,27,28 The increased incidence in agricultural workers may be attributed to exposure to a variety of agents, including organochlorines, organophosphates, and phenoxyacid herbicides.21,22 Despite many studies, an association with lymphoma incidence with herbicide or pesticide exposure has not reached the level of scientific certainty.21 Indeed, evidence indicates that modest exposure to herbicides by adults who use such products in and around their homes is not likely to increase lymphoma risk,35 although heavy occupational exposures remain under study. Large studies of two pesticides, chlorpyrifos36 and glyphosate37 showed no association with lymphoma incidence. In the former case, the relative risk of death from all causes, from any cancer, or from lymphoma was significantly less in the 22,000 applicators exposed to chlorpyrifos than in the 33,000 applicators not so exposed. It is possible that subsets of individuals may be susceptible to different exposures based on genetic differences (see “Interaction of Environment and Genotype” below) Indeed, preliminary studies using single nucleotide polymorphism–based analysis have linked lymphomagenesis with normal variations (polymorphisms) in genes involved in apoptosis, cell cycle regulation, lymphocyte development, and inflammation.38,39,40 In some cases, polymorphic genes have been associated with specific morphologic subsets of lymphoma.41 Dark hair dyes have also been associated with a moderately increased risk of follicular lymphoma in women.42 A meta-analysis has demonstrated a dose–response relationship of NHL with cigarette smoking, with increasing NHL risk in heavy or long-duration smokers, possibly from a suppressive effect of smoking on the immune system.20 Increased body mass index is associated with an increased risk of many cancers,43,44 including lymphoma.44,45,46,47 Indexes above 30 to 35 kg/m2 (normal = 8.5 to 25 kg/m2) are associated with an increased risk of lymphoma and a worse outcome after treatment. The risk factors discussed in this section have not been found uniformly in all studies of their association with lymphoma and should be considered provisional associations. In addition, as larger populations of patients with lymphoma, stratified by histologic type, are studied, it may become possible to dissect out etiologic relationships of exogenous exposures with specific subtypes of lymphoma but not others.
Small but significant increases in lymphoma are associated with radiation exposure. An increased incidence of lymphoma was reported in survivors of the atomic bombings in Hiroshima and Nagasaki who were near the hypocenter of the detonation.48,49,50,51 An increased incidence of lymphomas also has been reported for individuals at the Chernobyl accident site who received radiation equivalent to that of the atomic bomb exposures in Japan.52 Individuals treated a half century ago with radiation for ankylosing spondylitis also had a small increase in lymphoma incidence.53 The relative risk of lymphoma with high-dose radiation is low and some still question the causal relationship.54 Several studies have found an inverse relationship between an individual’s exposure to ultraviolet light and lymphoma incidence (especially DLBCL).55
Polymorphic immune gene variations have been identified as significant factors in the association of organochloride exposure with increased incidence of lymphoma in a large population of patients and matched controls.56 Associations between all exposures and NHL risk were limited to the same genotypes for interferon-γ, IFNG (C-1615T) TT, and interleukin-4, IL4 (5′-UTR, Ex1–168C→T) CC. Associations between PCB180 in plasma and dust and NHL risk were limited to the same genotypes for interleukin-16, IL16 (3′-UTR, Ex22+871A→G) AA, interleukin-8, IL8 (T-251A) TT, and interleukin-10, IL10 (A-1082G) AG/GG. This result indicates that the relation between organochlorine exposure and NHL risk may be modified by particular variants in immune genes, supporting the concept of a gene-environment interaction for induction of NHL.
Adult T-cell leukemia/lymphoma (ATLL) provides the most compelling evidence for a viral etiology of lymphoma.57 Human T-cell leukemia/lymphoma virus-1 (HTLV-1) is a C-type RNA tumor virus, isolated from patients with ATLL.58 HTLV-1 is an acquired retrovirus that is not related to other known animal retroviruses. HTLV-1 can immortalize lymphoid cells in culture and induce malignancy in an infected human host. The incidence of infection with HTLV-1 in endemic areas is very high, yet few of these infected patients develop ATLL. HTLV-1 also leads to a neurologic disorder called tropical spastic paraparesis.59 Host determinants affect transformation of lymphocytes by HTLV-1, and these may be genetic factors.58 Development of ATLL is associated with infection by the virus.60 Serum specimens from Japanese patients with ATLL are positive for HTLV-1, as are serum samples from ATLL patients in the Caribbean, where ATLL is endemic.61 The highest prevalence of ATLL in Japan is in the southern island of Kyushi, where 10 to 15 percent of the population has antibody to HTLV-1.62 On the Japanese islands where ATLL is rare, the rate is less than 1 percent. These and additional data from the Caribbean, the southeastern United States, South America, and Africa indicate that ATLL clusters in regions where HTLV-1 is prevalent.60,61 How these regions are linked is not known. One hypothesis is that HTLV-1 was brought to the Americas from Africa by the slave trade and then to the southern islands of Japan by trade between Japan and Africa.61,63
Host susceptibility, a shared environmental exposure, or both contribute to HTLV-1 infection. The prevalence of HTLV-1 antibodies in close family members is three to four times higher than in the corresponding normal population.63,64 In some instances, cell cultures of antibody-positive, clinically normal patients yield HTLV-1 isolates.64 Blood donors are routinely screened for antibodies to HTLV-1 to prevent transmission by this route.
Some B-cell lymphomas, including Burkitt lymphoma, posttransplantation lymphoma, and HIV-associated lymphomas (immunodeficiency-related Burkitt lymphoma, primary central nervous system lymphoma, primary effusion lymphoma, the immunoblastic-plasmacytoid type of DLBCL, and oral cavity plasmablastic lymphoma) may be caused by Epstein-Barr virus (EBV; Chaps. 98 and 102).65 EBV is a DNA virus in the herpesvirus family that first was described in cultured lymphoblasts from patients with African Burkitt lymphoma.66 EBV binds to the CD21 antigen (also the receptor for the C3d component of complement) on B lymphocytes.67 It is capable of transforming B lymphocytes into lymphoblastoid cells that may proliferate perpetually in cell culture.68 EBV is present in greater than 95 percent of cases of endemic Burkitt lymphoma and in approximately 20 percent of cases of nonendemic Burkitt lymphoma.69,70 Malaria is holoendemic in regions where endemic Burkitt lymphoma exists.71 A three-step process in the development of this lymphoma has been proposed72,73: (1) EBV initiates a polyclonal proliferation of B cells; (2) malaria or other infections further stimulate the proliferating B cells; and (3) the transforming B cells incur specific reciprocal translocations of chromosome 8 with chromosome 2, 14, or 22, resulting in a clonal expansion of B lymphocytes.
Extranodal NK/T-cell lymphoma, nasal type is mostly endemic to East Asia and is usually associated with EBV infection (Chap. 104). The EBV genome is typically detected in the lymphoma cells.74,75 Geographic localization of extranodal NK/T-cell lymphoma matches the endemic distribution of EBV, suggesting the role of EBV in lymphomagenesis.
Human herpesvirus-8 (HHV-8) is associated with Kaposi sarcoma, Castleman disease, and primary effusion lymphoma, found most commonly in immunodeficient individuals infected with HIV.65,76,77,78,79 HHV-8 is not a ubiquitous virus. It is mainly endemic in areas where classical or Kaposi sarcoma is of high prevalence, including the Mediterranean basin and East and Central Africa. In the latter areas, the HHV-8 seroprevalence can reach 80 percent in the adult population.79 In the homosexual population (mainly in the United States and Europe), HHV-8 is principally transmitted during repeated sexual contacts, whereas in Africa it is mainly transmitted from mother to child and among siblings. Saliva seems to play a major role in HHV-8 transmission.79 Posttransplantation primary effusion lymphoma is associated with HHV-8.76
Hepatitis B and C have been implicated in the pathogenesis of lymphoproliferative diseases.80 In one study, 334 newly diagnosed lymphoma patients and 1014 controls had a serologic evaluation for the presence of prior hepatitis virus B or C infection.81 The results suggested that hepatitis B seropositivity was significantly higher in patients with DLBCL and follicular lymphoma, and seropositivity for hepatitis C was significantly higher in DLBCL patients. A similar result was found in another study in Taiwan, an area with a high frequency of hepatitis B virus infection.82 In two other studies, seropositivity for hepatitis C was significantly higher in patients with B-cell lymphomas.83,84 Hepatitis C virus has a predilection for B cells. Hepatitis C virus RNA levels are significantly higher in B cells than CD4+ or CD8+ T cells or other cells in infected patients, and the virus is associated with immunopathologic reactions, such as cryoglobulinemia, and, not infrequently, clonality of infected B lymphocytes.85 Hepatitis C virus infection may be associated with the onset of DLBCL, marginal zone lymphoma, and lymphoplasmacytic lymphoma, but not follicular lymphoma.83
Helicobacter pylori can cause marginal zone B-cell MALT lymphomas of the stomach and probably causes some of the higher-grade lymphomas, either from transformation of a MALT or de novo large cell lymphoma.86,87,88 This spiral Gram-negative bacillus is the first bacterium demonstrated to cause a human neoplasm. It had been thought that the stomach was sterile because of the acid environment, but H. pylori had evolved to tolerate the environment, perhaps in part, because it secretes urease, an enzyme that converts urea to ammonia, making the microenvironment around the organism less acidic. Although the stomach has no endogenous lymphoid tissue, the latter develops in response to the organism, and ultimately the chronic inflammatory reaction can result in the transformation and selection of a mutant lymphocyte with a growth and survival advantage leading to a lymphoma (Chap. 101). Eradication of H. pylori with antibiotics early in the course of gastric MALT lymphoma can lead to regression of the lymphoma and permanent cure of the majority of afflicted patients.89
Ocular adnexal lymphomas are the most common tumor of the eye.90,91 The majority of ocular adnexal lymphomas are extranodal, mucosa-associated lymphoid tissue lymphomas and have been linked to Chlamydophila psittaci infection in several reports. In one study, this organism was detected in lymphoma tissue in 75 percent of cases.92 DNA was detected in conjunctional swabs and/or blood mononuclear cells from 50 percent of patients. Mononuclear phagocytes were the carriers of C. psittaci in this population of patients.93 Confirmatory reports of the association of C. psittaci with adnexal ocular lymphoma have been published,94,95 but other studies report no association.96,97,98 These discrepant reports may be explained by the association of different organisms with ocular lymphoma in different geographic regions of the world.99,100
Other bacterial infestations have been found in association with lymphomas of MALT. Campylobacter jejuni and Borrelia burgdorferi have been connected to the onset of immunoproliferative disease of the small intestine and B-cell lymphoma of the skin.101
A number of the rare immunodeficiency syndromes tabulated in Table 95–282,102–123 result from gene mutations leading to deficiencies in cellular or humoral immunity or both. These syndromes have a paradoxically high frequency of autoantibodies but, more relevant to this discussion, an increased probability of developing a lymphoma. Because these syndromes are so uncommon, reliable assessment of the increased risk of lymphoma often has to be inferred. The increased risk of approximately 0.5 to 10 percent of patients, depending on the immunodeficiency disease in question, is several orders of magnitude above the risk in the general population (age-adjusted incidence rate in the United States younger than 65 years of age: males = 0.011 percent and females = 0.008 percent). With the exception of common variable immunodeficiency, the syndromes present in childhood and because several are X-chromosome-linked, males are affected more commonly than females. The lymphomas induced may result from a susceptibility to EBV and the lymphoproliferation may initially be polyclonal before evolving to a monoclonal tumor. Extranodal involvement appears to be more common than in persons with lymphoma who are immunocompetent. The initial manifestations of the immunodeficiency are usually infections or autoimmune abnormalities, such as immune cytopenias, and lymphoma is a later complication. We have included Li-Fraumeni syndrome in this cluster for convenience of presentation. This germline predisposition syndrome does not have an immunodeficiency phenotype as do all the other entries in Table 95–2. Rather, it is a nonsyndromic familial cancer syndrome transmitting susceptibility to mutations in p53. The cancers that occur in these families include lymphoma.
| Altered Genes | |||||
|---|---|---|---|---|---|
| Syndrome | Inheritance | Description | Mechanism | Leukemia Type | References |
| DNA REPAIR DEFECTS | |||||
| Ataxia telangiectasia | R | ATM homozygotes Dominant-negative missense mutations | Genomic instability Increased translocations in T cells formed at the time ofV(D)J recombination | T-cell lymphoma, T-cell ALL, T-cell PLL, B-cell lymphoma | 112,115 |
| Bloom | R | BLM | Genomic instability | ALL, lymphoma | 107,108 |
| Nijmegen breakage | R | NBS1 | Genomic instability Altered telomere maintenance | Lymphoid tumors, especially B-cell lymphoma | 103,109 |
| TUMOR-SUPPRESSOR GENE DEFECT | |||||
| Li-Fraumeni* | D | p53 | Defect in tumor suppressor | CLL, ALL, Hodgkin and Burkitt lymphoma | 111,120 |
| IMMUNODEFICIENCY STATES | |||||
| Common variable immunodeficiency | R and D | Defect in CD40 signaling | Failure of B-cell maturation | Burkitt, MALT, other B-cell lymphomas, Hodgkin lymphoma | 102,240 |
| Severe combined immunodeficiency disease (SCID) | R | ADA | Defective T-cell + B-cell function | B-cell lymphoma | 113 |
| Wiskott-Aldrich | X | WASP | Signaling and apoptosis | Hodgkin and non-Hodgkin lymphoma | 117,119 |
| X-linked immunodeficiency with normal or increased immunoglobulin (Ig) M | X | CD40L | CD40 ligand defect on T cell | Hodgkin and non-Hodgkin lymphoma | 116,123 |
| X-linked lymphoproliferative syndrome (XLP) | X | SAP | Defect in immune signaling | EBV-related B-cell lymphoma | 110 |
| APOPTOTIC DEFECT | |||||
| Autoimmune lymphoproliferative syndrome (ALPS) | D | APT (FAS) | Germline heterozygous FAS mutations; defective apoptosis | Lymphoma | 106,241 |
| UNKNOWN DEFECT | |||||
| Dubowitz | R | Unknown | Unknown | ALL, lymphoma | 105 |
| Poland | D | May not be inherited | Unknown | ALL, lymphoma | 104,114,118 |
| Wilms tumor (WT) | D | Unknown | Unknown | ALL, Castleman disease | 122 |
A variety of types of immunosuppressed individuals develop lymphoma. Chapter 81 discusses AIDS-related lymphoma.124 Posttransplantation lymphoproliferative diseases generally display B-cell lineage derivation, involvement of extranodal sites, aggressive histology and clinical behavior, and frequent association with EBV infection. The occurrence of immunoglobulin (Ig) V mutations in the overwhelming majority of posttransplantation lymphoproliferative disease indicates that malignant transformation targets germinal center B cells and their descendants, both in EBV-positive and EBV-negative cases.125,126,127 Posttransplantation T-cell lymphomas may occur and often arise in extranodal sites, such as skin or central nervous system.128,129
The incidence and severity of lymphomas have increased with the introduction of immunosuppressive agents such as cyclosporine, infliximab, and etanercept for treatment of autoimmune diseases.
Several autoimmune disorders are risk factors for lymphoma, probably as a result of chronic immune stimulation leading to excessive B-cell proliferation as well as depressed regulatory T-cell function. The strongest associations are for primary Sjögren syndrome, systemic lupus erythematosus, and rheumatoid arthritis with relative risks of 18.8, 7.4, and 3.9, respectively.20 Patients with Sjögren syndrome are especially susceptible to development of parotid gland marginal zone lymphoma (1000-fold increased risk), DLBCL and follicular lymphomas (Fig. 95–3). Similarly, marginal zone lymphoma and DLBCL are the subtypes most likely to develop in patients with lupus.130
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree