Food Allergies
John James
Wesley Burks
Introduction
Valuable information concerning the basic science and clinical aspects of food allergy and other adverse reactions to foods has continued to be published over the past decade. The term adverse food reaction encompasses a variety of reactions to food, only some of which are the result of IgE-mediated food allergy. In an attempt to standardize nomenclature in the scientific literature, the American Academy of Allergy and Immunology and the National Institutes of Health have defined adverse food reactions (1). An adverse food reaction is defined as any untoward reaction to a food or food additive following ingestion. These reactions can be further subdivided into food allergy and food intolerance. Basically, food allergy is any adverse food reaction due to an immunologic mechanism (e.g., IgE-mediated and non-IgE-mediated). In contrast, food intolerance is any adverse reaction due to a nonimmunologic mechanism. These reactions may be the result of pharmacologic properties of the food (e.g., caffeine in irritable bowel syndrome; tyramine-induced nausea, emesis, and headache in patients with migraines), toxins in the food, usually from improper food handling (e.g., histamine generation in scombroid fish poisoning; bacterial food poisoning), foods that exacerbate reflux (peppermint, spicy or acidic food) or metabolic disorders (lactase deficiency; pancreatic insufficiency) (Table 18.1). This chapter will summarize several key areas related to food allergy including epidemiology, pathophysiology and mucosal immunity, food allergens and IgE, as well as non-IgE-mediated food reactions. Other areas that will be reviewed include the diagnosis, management, and treatment of food allergy; a review of the natural history of food allergy; and exciting new developments in food allergy treatment.
Table 18.1 Food Allergy: Differential Diagnosis | |
|---|---|
|
Epidemiology
The true incidence and prevalence of food allergy has been difficult to determine in the past. Despite this, significant progress has been made over the past 10 years. Recent epidemiological studies suggest that nearly 4% of Americans are afflicted with food allergy (1) and children are more affected than adults. Using a randomized telephone survey and standardized questionnaire in 10 European nations, an approximate measure for food allergy prevalence was determined to be 3.75% with the most affected age group being 2- to 3-year-olds (2). Unfortunately, the overall prevalence of food allergy has traditionally been overestimated. For example, 28% of mothers in one investigation perceived their children to have had at least one adverse reaction to food (3), but only 8% or one-third of these children had reactions confirmed by double-blind, placebo-controlled food challenges (DBPCFC). A recent meta-analysis reviewed the prevalence of food allergy according to the method of assessment used (5). The prevalence of self-reported food allergy was very high, 3% to 35% for any food, compared with objective measures. There was a marked heterogeneity in the prevalence of food allergy that could be a result of differences in study design or methodology, or differences among populations.
Within the past few years, specific investigations have examined the prevalence rates of allergy to specific foods such as peanuts and seafood, which typically can be severe, lifelong, and potentially fatal allergies. First, in North America and the United Kingdom, the prevalence rates among schoolchildren are now in the excess of 1% (6). Second, physician-diagnosed and/or convincing seafood allergy has been reported by 2.3% of the general population, or approximately 6.6 million Americans (7).
The epidemiology of food allergy can certainly be influenced by the specific disease state (Table 18.2). For example, the prevalence of food allergy appears to be approximately 30% in children with moderate-severe, refractory atopic dermatitis (8). Likewise, an Australian investigation determined the relative risk of an infant with atopic dermatitis having an IgE-mediated food allergy was 5.9% for the most severely affected group (9).
Table 18.2 Food Allergy Prevalence in Specific Disorders | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Studies from the UK have determined the cumulative incidence of food hypersensitivity by 12 months to be 4% (95% confidence interval (CI), 2.9% to 5.5%) on the basis of open food challenges and 3.2% (95% CI,
2.2% to 4.5%) on the basis of DBPCFC (10). The rate of parentally perceived food hypersensitivity was considerably higher than objectively assessed food hypersensitivity. In addition, the incidence of food allergy was found to be 5% to 6% in children by 3 years of age based on food challenges and a good clinical history (11).
2.2% to 4.5%) on the basis of DBPCFC (10). The rate of parentally perceived food hypersensitivity was considerably higher than objectively assessed food hypersensitivity. In addition, the incidence of food allergy was found to be 5% to 6% in children by 3 years of age based on food challenges and a good clinical history (11).
Pathophysiology/Mucosal Immunity
Mucosal Barrier
The main function of the gastrointestinal tract is to process ingested food into a form that can be absorbed and utilized for energy and cell growth. This process requires that the intestinal immune system be capable of discriminating between harmful and harmless foreign proteins (12). Both nonimmunologic and immunologic mechanisms help to block harmful foreign antigens (bacteria, viruses, parasites, food proteins) from entering the interior of the body, thus forming the gastrointestinal “mucosal barrier.” The developmental immaturity of these mechanisms in infants reduces the efficiency of the infant mucosal barrier, and likely plays a major role in the increased prevalence of gastrointestinal infections and food allergy seen in the first few years of life. The relatively low concentrations of (secretory) S-IgA in the infant’s intestine and the relatively large quantities of ingested proteins place a significant burden on the immature gut-associated immune system.
The gut-associated lymphoid tissue (GALT) must mount a significant response against potentially harmful foreign substances and pathogenic organisms, but must remain unresponsive to enormous quantities of nutrient antigens and about 1014 commensal organisms forming the normal gut flora. The GALT is comprised of four distinct lymphoid compartments: (a) Peyer’s patches (PPs) and the appendix (aggregates of lymphoid follicles throughout the intestinal mucosa), (b) lamina propria lymphocytes and plasma cells, (c) intra-epithelial lymphocytes interdigitated between enterocytes, and (d) mesenteric lymph nodes (12).
S-IgA, a dimeric form of IgA that is found in intestinal secretions, does not activate complement or bind to Fc receptors, and therefore does not induce inflammatory responses. S-IgA antibodies are directed against bacterial or viral surface molecules that can prevent their binding to the epithelium and/or facilitate agglutinating pathogens resulting in complexes that become trapped in the mucus barrier and pass out in the stool (13). Despite the evolution of this well developed barrier system, about 2% of ingested food antigens are absorbed and transported throughout the body in an “immunologically” intact form, even through the mature gut (14).
Oral Tolerance Induction
Oral tolerance is defined as the specific immunological unresponsiveness to antigens induced by their prior feeding (12). Intact food antigens penetrate the gastrointestinal tract and enter the circulation in both normal children and adults (15,16). These intact proteins do not normally cause clinical symptoms because most individuals develop tolerance to ingested antigens. In mucosal tissues, soluble antigens, such as food and inhaled antigens are usually poor immunogens, inducing a state of unresponsiveness known as oral tolerance. Unresponsiveness of T cells to ingested food proteins may be the result of three different mechanisms: induction of regulatory T cells, T-cell clonal deletion, or T-cell anergy.
Commensal gut flora take residence in the gastrointestinal tract within 24 hours of birth at a concentration that has been estimated to be between 1012 and 1014 bacteria per gram of colon tissue (17). The impact of gut flora on oral tolerance is supported by the finding that mice raised in germ-free environments following birth are unable to develop tolerance to orally administered ovalbumin (18).
Normal Immune Response to Ingested Antigens
Low concentrations of detectable serum IgG, IgM, and IgA food-specific antibodies are commonly found in normal individuals (19). In general, the younger an infant when a food antigen is introduced into the diet, the more pronounced the antibody response (20). Following the introduction of cow’s milk, serum milk protein-specific IgG antibodies rise over the first month, achieving peak antibody levels after several months, and then generally decline, even though cow’s milk proteins continue to be ingested (21). Individuals with various inflammatory gastrointestinal disorders (e.g., celiac disease, food allergy, inflammatory bowel disease) frequently have high levels of food-specific IgG and IgM antibodies. It is important to recognize that these antibodies do not indicate that the patient is allergic to these foods (22). The increased levels of food-specific antibodies (not IgE) appear to be secondary to increased gastrointestinal permeability to food antigens and simply reflect dietary intake.
Allergens
Sensitization to food allergens may occur in the gastrointestinal tract, considered “traditional” or Class 1 food allergy, or as a consequence of an allergic sensitization to inhalant allergens, considered Class 2 food allergy (23). The major food allergens that have been identified in Class 1 allergy are water-soluble glycoproteins that have molecular weights ranging from 10 kilodaltons to 70 kilodaltons (kD) and are stable to treatment with heat, acid, and proteases (24) (Table 18.3). There are no consistent physicochemical properties common to the Class 2 food allergens. The majority of these generally plant-derived proteins are highly heat-labile and difficult to extract. A number of the Class 1 and 2 food allergens have been identified, cloned, sequenced, and expressed as recombinant proteins. Many of the plant-related allergens are homologous to pathogen-related proteins (PRs), which are expressed by the plant in response to infections or other stress factors, or comprise seed storage proteins, profilins, peroxidases, or protease inhibitors common to many plants (25).
Table 18.3 Allergens | |
|---|---|
|
Cow’s Milk
Cow’s milk is the most common food allergy in young children (if both IgE- and non-IgE-mediated hypersensitivities are considered) (1). IgE-mediated cow’s milk allergy affects 2.5% of children less than 2 years of age and most patients become tolerant by 4 to 5 years of age. Cow’s milk contains at least 20 protein components, which may lead to antibody production in man (26). The milk protein fractions are subdivided into casein (76% to 86%) and whey proteins. The casein fraction is precipitated from skim milk by acid at pH 4.6 and is comprised of four basic caseins (αs1, αs2, β, and κ comprising 32%, 10%, 28%, and 10% of the total milk protein, respectively). The noncasein fraction, or whey, consists of β-lactoglobulin, α-lactalbumin, bovine immunoglobulins, bovine serum albumin, and minute quantities of various proteins (e.g., lactoferrin, transferrin, lipases, esterases). Extensive heating will destroy several of the whey proteins. However, routine pasteurization is not sufficient to denature these proteins and has been reported to increase the allergenicity of some milk proteins, such as β-lactoglobulin (27). Sequential (linear) allergenic (IgE) epitopes have been mapped on the caseins, β-lactoglobulin and α-lactalbumin, and have been correlated with the persistence of cow’s milk allergy (28–30). The most recent studies with more highly purified proteins suggest that the casein proteins are more allergenic (31).
IgE-immunoblotting techniques also have shown cross-reactivity among milk proteins in cows, goats, and sheep, due to the high degree of homology among these proteins. Oral challenge studies in cow’s milk-allergic children indicated that at least 90% of cow’s milk-allergic children will react to goat’s milk (32). Interestingly, about 10% of milk-allergic children will react to beef, with a slightly higher number reacting to rare beef (33).
Chicken Egg
Chicken egg is another common IgE-mediated food allergy in children. The yolk is considered less allergenic than the white (34). The egg white contains 23 different glycoproteins with ovomucoid, ovalbumin and ovotransferin being identified as the major allergens (35,36). Although ovalbumin comprises the majority of the protein in egg white, ovomucoid has been shown to be the dominant allergen when highly purified egg white proteins are utilized (37). Ovomucoid (Gal d 1) is comprised of 186 amino acids arranged in three tandem domains, a set tertiary structure, and six sequential (linear) IgE-binding sites. Blinded oral food challenges with ovomucoid-depleted egg white demonstrated that
ovomucoid is responsible for clinical reactivity in the vast majority of egg allergic children (38). In addition, it was shown that about 70% of egg-allergic children may be able to ingest small amounts of egg protein in extensively heated (baked) products, e.g., breads, cakes, and cookies (39). These children appear to lack IgE antibodies to continuous epitopes, and since the prolonged high temperature destroys the tertiary structure (discontinuous or conformational epitopes) of the egg white proteins, the children fail to react (36,37).
ovomucoid is responsible for clinical reactivity in the vast majority of egg allergic children (38). In addition, it was shown that about 70% of egg-allergic children may be able to ingest small amounts of egg protein in extensively heated (baked) products, e.g., breads, cakes, and cookies (39). These children appear to lack IgE antibodies to continuous epitopes, and since the prolonged high temperature destroys the tertiary structure (discontinuous or conformational epitopes) of the egg white proteins, the children fail to react (36,37).
Peanuts
The peanut, a member of the legume family, is the most common food allergy in individuals beyond the age of 4 years in most industrialized societies (40). Peanut proteins are traditionally classified as albumins (water-soluble) and globulins (saline-solution soluble), the latter of which is further subdivided into arachin and conarachin fractions (36). Three proteins with molecular weights of 63.5 kD (Ara h 1) (34, 41), 17 kD (Ara h 2) (42), and 64 kD (Ara h 3) (43) have been identified as major allergens. Ara h 4–8 have also been identified (44). Ara h 5 is a profilin, whereas Ara h 4 appears to be an isoform of Ara h 3, and Ara h 6 and 7 appear to be isoforms of Ara h 2. Ara h 8 is a member of the pathogenesis-related PR-10 family, primarily involved in pollen-associated food allergy (45). Ara h 1 belongs to the vicilin family of seed storage proteins, Ara h 2 is a member of the conglutin family of storage proteins (46), and Ara h 3 is a member of the glycinin family of storage of proteins (43). Refined peanut oil was found to be safe in all 60 peanut allergic individuals, whereas pressed (or extruded) oils were found to retain some of their allergenicity (47).
Soybean
Soybean is another member of the legume family that provokes a significant number of hypersensitivity reactions predominantly in infants and young children. Since soybeans provide an inexpensive source of high quality protein, soybean protein is used in many commercial foods. Approximately 10% of the seed proteins are water-soluble albumins and the remainder are salt-soluble globulins. Four major protein fractions have been separated by ultracentrifugation: 2S (contained in whey fraction), 7S (50% β-conglycinin), 11S (glycinin), and 15S (aggregated glycinin). A number of soy proteins have been isolated and characterized, particularly a 34 kD thiol protease-like protein (Gly m Bd 30K). Interestingly, the allergenic epitopes on glycinin G1 acidic chain are homologous to IgE-binding epitopes on peanut Ara h 3 (48). Similar to highly refined peanut oil, refined soy oil did not provoke allergic reactions to soy in any of eight patients after ingesting up to 8 mL of soy oil (49).
Tree Nut
Tree nut allergies affect about 0.6% of the American population (50). In a national registry of peanut and nut allergic individuals, walnuts were the nut provoking the most allergic reactions (34%), followed by cashews (20%), almonds (15%), pecans (9%), and pistachios (7%). Hazel nuts, Brazil nuts, pine nuts, and macadamia nuts all account for less than 5%. Sesame seed allergy is becoming more commonly recognized in the United States (51,52). Skin testing reveals extensive cross-reactivity among tree nuts. While individuals allergic to one nut can clearly tolerate other nuts, too few patients have been systematically challenged to a variety of nuts to determine the extent of clinical cross-reactivity. Patients allergic to tree nuts do not necessarily need to avoid peanuts (a legume), and vice versa unless, for safety reasons, it is better to avoid all of them. However, surveys suggest that about 35% to 50% of peanut allergic patients are also reactive to at least one tree nut (53,54).
Fish
Fish are one of the most common causes of food allergic reactions in adults, and a common cause in children as well (7,55). The major allergen in cod, Gad c 1, is a parvalbumin that has been isolated from the myogen fraction of the white meat. It is heat-stable and resistant to proteolytic digestion, has a molecular weight of 12 kD, an isoelectric point of 4.75, and is composed of 113 amino acids (56). The 3-dimensional structure of Gad c 1 has been defined and shown to be arranged in 3 domains, 2 of which bind calcium (57). Unlike many other food allergens, the fish protein fraction(s) responsible for clinical symptoms in some patients appear to be more susceptible to manipulation (e.g., heating, lyophilization) than other foods, since reactions occurred during open feedings of the fish in approximately 20% of those with negative DBPCFCs utilizing lyophilized fish (58). Furthermore, it was found that most patients allergic to fresh cooked salmon or tuna could ingest canned salmon or tuna without difficulty, indicating that preparation led to destruction of the major allergens. Nevertheless, allergic reactions following exposure to airborne allergen emitted during cooking are not uncommon (57).
Shellfish
Shellfish allergens are considered a major cause of food allergic reactions in adults, affecting up to 2.3% of the U.S. adult population (7). This group consists of a wide variety of mollusks (snails, mussels, oysters, scallops, clams, squid, and octopus) and crustacea (lobsters, crabs, prawns, and shrimp). Shrimp allergens have been most extensively studied. Tropomyosin, a protein found both in muscle and elsewhere, has been identified as
the major allergen in shrimp (59). Considerable cross-reactivity among crustacea has been demonstrated by skin test and in vitro IgE analyses (60). Invertebrate tropomyosins are highly homologous and tend to be allergenic: crustaceans (e.g., shrimp, crab, crawfish, and lobster), arachnids (house dust mites); insects (cockroaches); and mollusks (squid, snails) (61). Vertebrate tropomyosin tends to be nonallergenic.
the major allergen in shrimp (59). Considerable cross-reactivity among crustacea has been demonstrated by skin test and in vitro IgE analyses (60). Invertebrate tropomyosins are highly homologous and tend to be allergenic: crustaceans (e.g., shrimp, crab, crawfish, and lobster), arachnids (house dust mites); insects (cockroaches); and mollusks (squid, snails) (61). Vertebrate tropomyosin tends to be nonallergenic.
Wheat
Wheat (spelt) and other cereal grains share a number of homologous proteins and are not infrequently implicated in food allergic reactions in children. It has been suggested that the globulin and glutenin fractions are the major allergenic fractions in antibody-mediated reactions (e.g., gliadins in celiac disease and albumins in Baker’s asthma) (62). Nonspecific binding to lectin fractions has been noted with each grain, and extensive immunologic cross-reactivity has been reported among the cereals, as was corroborated with skin-prick testing. In addition, homologies to allergenic proteins in grass pollens account for a large number of clinically irrelevant positive skin tests to wheat and other cereal grains. More recent studies have suggested that the water-insoluble gliadin fraction may be important in clinical reactivity to wheat, especially in cases of food-associated exercise-induced anaphylaxis (63–65).
Pathogenesis-related Proteins
Pathogenesis-related proteins (PRs) have been shown to comprise a large number of Class 2 allergenic proteins found in various vegetables and fruits (Table 18.4) (23,66,67). These proteins are induced when pathogens stress the plant; examples include wounding or certain environmental stresses, such as drought and heat. PRs have been classified into 14 families, although 6 PR families account for the majority of cross-reactivity among plant proteins. Two families of chitinases that are similar to the latex allergen, Hev b 6.02, have been identified as allergens in a number of vegetables: PR-3 type proteins are found in chestnut and avocado (Pers a 1) while PR-4 type proteins are wound-induced proteins found in tomato and potato (Win 1 and Win 2 proteins). PR-5 type thaumatin-like proteins have been identified as cross-reacting proteins found in apples (Mal d 2) and cherry (Pru av 2). The PR-10 type proteins are homologous to the major birch pollen allergen, Bet v 1, and account for cross-reactivity between birch pollen and fruits of the Rosaceae species: apple (Mal d 1), cherry (Pru av 1), apricot (Pru ar 1), pear (Pyr c 1); or vegetables of the Apiaceae species: carrot (Dau c 1), celery (Api g 1), and parsley (pcPR 1 and 2); and hazel nut (Cor a 1). The lipid transfer proteins (LTPs), or PR-14 type proteins, form a family of 9 kD proteins distributed widely throughout the plant kingdom. LTPs have been identified as major allergenic proteins in Prunoideae species such as peach (Pru p 1 in peach skin and Pru p 3 in the fruit), apple (Mal d 3), apricot, plum, and cherries. Gly m 1, a major allergen in soybean, has been found to be a LTP.
Table 18.4 Pathogenesis-related Proteins | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Profilin
Profilin is an actin-binding protein that was first identified in birch pollen (Bet v 2) and is now recognized as an allergenic protein in a number of fruits and vegetables (23). Profilins are responsible for the celery-mugwort-spice syndrome and is responsible for oral allergy syndrome to apple, pear, carrot, celery (Api g 4), and potato in birch pollen-allergic patients. Profilins have also been identified in tomato, peanut (Ara h 5), and soybean (Gly m 3), but whether these proteins cause allergic reactions remains to be established.
IgE-Mediated Food Reactions
Immune responses mediated by specific IgE antibodies to food allergens are the most widely recognized mechanism for food-induced allergy symptoms (1). Atopic patients produce IgE antibodies to specific epitopes in the food allergen. These antibodies bind to high affinity
IgE receptors on basophils and tissue mast cells throughout the body. When antigen binds to multiple adjacent IgE antibodies on a mast cell or basophil, these cells become activated, degranulate, and release preformed mediators such as histamine and newly formed mediators such as leukotrienes and prostagladins. These mediators are responsible for the immediate allergic reaction and the clinical symptoms observed. Eosinophils, monocytes, and lymphocytes are recruited to the area affected in the late-phase allergic response and release a variety of cytokines and inflammatory mediators. Mast cell-derived mediators can cause endothelial cells to increase expression of adhesion molecules for eosinophils, basophils, and lymphocytes. The following section will review the specific clinical manifestations of IgE-mediated food reactions.
IgE receptors on basophils and tissue mast cells throughout the body. When antigen binds to multiple adjacent IgE antibodies on a mast cell or basophil, these cells become activated, degranulate, and release preformed mediators such as histamine and newly formed mediators such as leukotrienes and prostagladins. These mediators are responsible for the immediate allergic reaction and the clinical symptoms observed. Eosinophils, monocytes, and lymphocytes are recruited to the area affected in the late-phase allergic response and release a variety of cytokines and inflammatory mediators. Mast cell-derived mediators can cause endothelial cells to increase expression of adhesion molecules for eosinophils, basophils, and lymphocytes. The following section will review the specific clinical manifestations of IgE-mediated food reactions.
Cutaneous Manifestations
Cutaneous manifestations are the most common clinical symptoms of food allergy (68). These cutaneous symptoms range from acute urticaria or angioedema to a morbilliform pruritic dermatitis. Chronic urticaria is almost never caused by food allergy (69). Cutaneous symptoms may present with signs and symptoms from other organ systems such as the gastrointestinal and respiratory systems (70). Urticaria can be elicited in approximately 12% of food challenges and overall, the incidence of acute food-dependent urticaria is about 1% to 2% (70). Contact dermatitis also has been reported to various foods (71). True allergic contact urticaria can proceed to a systemic reaction; therefore, a thorough diagnostic workup to rule out involvement of the immune system is important. In children with atopic dermatitis, food allergies have been confirmed by DBPCFC in about one-third of the children (72,73). In one study of 210 children evaluated and followed to determine a relationship between food allergy and exacerbations of their atopic dermatitis, 62% of children had a reaction to at least one food. Of all reactions that occurred within 2 hours of a DBPCFC, 75% were cutaneous (74). Cutaneous manifestations predominantly involved erythema and pruritus leading to scratching and exacerbation of the atopic dermatitis.
Sampson and Broadbent reported an increase in histamine releasabililty in patients with atopic dermatitis who repeatedly ingest a food allergen (75). This is probably due to the stimulation of mononuclear cells to secrete histamine-releasing factors (HRFs), some of which interact with IgE molecules bound to the surface of basophils. Increased HRF production has been associated with an increase in symptoms as well as increased lung and skin hyperreactivity.
Gastrointestinal Manifestations
Gastrointestinal symptoms are the second most frequently observed manifestation of food allergy. Clinical presentations include nausea, vomiting, diarrhea, and abdominal pain and cramping. As with cutaneous manifestations, gastrointestinal symptoms may occur alone or in combination with symptoms from other organ systems. There is considerable evidence that many of these symptoms result from the activation of mast cells (76).
Allergic eosinophilic gastroenteropathies are manifested by eosinophilic infiltration of the gastrointestinal tract. Symptoms depend on the layers of GI tract involved and are intermittent (77). Often this is associated with peripheral eosinophilia but rarely involves other organs. These patients do not meet criteria for hypereosinophilic syndrome (78). Eosinophilic infiltration of the mucosal layer is most common and can be seen in any part of the GI tract. Clinical symptoms include abdominal pain, postprandial nausea, vomiting, diarrhea, weight loss, failure to thrive, occult or gross blood loss in the stools, anemia, hypoalbuminemia, and peripheral edema (77,79). Involvement of the submucosal and muscular regions is more common in the prepyloric region of the gastric antrum and the distal small intestine. These patients also may have symptoms of gastric outlet obstruction, a mass lesion with epigastric tenderness, and even perforation of the intestinal wall (78,80). Rarely, eosinophilic infiltration involves the serosal surface, presenting with prominent ascites (78,79).
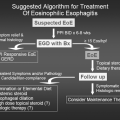
Over the past few years, there has been a dramatic increase in the number of publications related to eosinophilic esophagitis (EE) (81,82). Chapter 40 is devoted to EE and gastroesophageal reflux disease (GERD) and discusses them in additional detail. Typical clinical symptoms for EE depend on the age group involved. For example, young children with EE usually present with GERD that has not responded well to typical medical management and who have normal pH probes results. In contrast, adolescents present with dysphagia and adults present with dysphagia, food impaction, and strictures (81). There may be a significant delay between symptoms and confirmatory diagnosis by esophageal biopsy, which typically reveals greater than 15 to 20 intramucosal esosinophils per high-power field, preferably with multiple biopsy sites (77). Food allergens have been implicated as causative agents in more than 90% of patients with EE. A combination of skin-prick testing and atopy patch testing have been utilized to identify offending food allergens in patients with this disease (77,81–85). The more common food allergens that have been implicated in EE include cow’s milk, eggs, soy, chicken, wheat, beef, peanuts, rice, and potatoes. The usefulness of the atopy patch test in the diagnostic workup of children with food allergy-related gastrointestinal symptoms is still unclear; its diagnostic accuracy appears to be higher with fresh foods than with commercial extracts (84). The medical management of EE has been investigated in detail (8). The three main dietary strategies that have been utilized and assessed include a strict elemental diet (e.g., amino
acid-based formula), an elimination diet based on the results of the skin-prick test and atopy patch test findings or removal of the six most common food allergens (e.g., cow’s milk, eggs, wheat, soy, nuts, and seafood). While up to 98% of patients experience significant improvement with a strict elemental dietary therapy, this response rate decreases with the other elimination diets (77). Another form of therapy that has been utilized in these patients involved the use of swallowed inhaled corticosteroid preparations and general guidelines have recommended (77,83).
acid-based formula), an elimination diet based on the results of the skin-prick test and atopy patch test findings or removal of the six most common food allergens (e.g., cow’s milk, eggs, wheat, soy, nuts, and seafood). While up to 98% of patients experience significant improvement with a strict elemental dietary therapy, this response rate decreases with the other elimination diets (77). Another form of therapy that has been utilized in these patients involved the use of swallowed inhaled corticosteroid preparations and general guidelines have recommended (77,83).
Allergic eosinophilic gastroenteritis is another eosinophilic gastroenteropathy that is less common than EE (78). This condition can present in infancy through adolescence and typical clinical symptoms include abdominal pain, nausea/vomiting, diarrhea, poor appetite, weight loss, occult blood in the stool, and in some cases, a protein-losing enteropathy. Approximately 50% of these patients are atopic and 50% have a peripheral eosinophilia. The most common form of this disease is characterized by endoscopic biopsy findings of a significant eosinophilic infiltration of the gastric mucosa and submucosa. Resolution of clinical symptoms can be observed with the removal of the causal food within 6 weeks, and the most common offending foods include cow’s milk, eggs, soy cereals, and fish. Oral cromolyn and ketotifen have been utilized with some success (77). Of note, most patients have an excellent response to use of an elemental diet such as an amino acid-based formula. While the clinical course can be prolonged, the natural history of this condition is not well understood (86).
Pollen-Food Syndrome (Oral Allergy Syndrome)
Patients allergic to certain airborne pollens can display adverse reactions on the ingestion of plant-derived foods as a result of IgE-cross-reactive structures shared by pollen and food allergen sources. This clinical entity is known as the pollen-food syndrome, PFS ( also known as the oral allergy syndrome) and it is considered to be a form of contact urticaria with symptoms resulting from interaction of the food allergen with the oral mucosa (87,88). Symptoms include pruritus with or without angioedema of the lips, tongue, palate, and posterior oropharynx. Rarely, systemic anaphylaxis can be observed with this syndrome. Shared allergen sensitivities have been reported between ragweed and the gourd family (watermelon, cantaloupe, honeydew melon, zucchini, and cucumbers) and bananas (89). PFS has been described with ingestion of apples (90), carrots, parsnips, celery, hazelnuts, potatoes (91,92), celery (93), and kiwi (94) in patients sensitive to birch pollen, and with ingestion of apples, tree nuts, peaches, oranges, pears, cherries, fennel, tomatoes, and carrots in patients allergic to tree and grass pollens (95). PFS symptoms resolve rapidly and rarely involve any other target organs. However, ingestion of celery tuber (celery root), which cross-reacts with birch pollen, may cause more severe systemic symptoms in pollen-allergic patients (93). This may be explained by the presence of both heat-labile and heat-stable proteins (96).
Respiratory Manifestations
Oral Ingestion of Food Allergens
Oral ingestion of food allergens is the primary route of exposure that can cause or exacerbate food hypersensitivity symptoms. Anaphylactic reactions to foods including significant respiratory symptoms and, in some cases, fatal and near fatal anaphylactic reactions have also been reported (68




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree