Fig. 24.1
A high-resolution ultrasonography images of (1) follicular adenoma (a–c), (2) Hürthle cell adenoma (d–f), (3) follicular thyroid carcinoma (g–i), and (4) Hürthle cell carcinoma (j–l)
Hürthle cell neoplasms have a wide range of sonographic appearances from predominantly hypoechoic to hyperechoic lesions with various degrees and patterns of vascularization. Ultrasonography cannot distinguish Hürthle cell adenoma from HCC, unless obvious local invasion or lymphadenopathy is seen. Common sonographic features of Hürthle cell neoplasms include a hypoechoic or isoechoic appearance, with heterogeneity. Cystic changes and sonographic halo signs are common. Coarse calcification occurs in 20 % of cases. Most HCC have internal and peripheral vascularity (Fig. 24.1) [21, 22].
Fine-Needle Aspiration Biopsy
Although fine-needle aspiration biopsy (FNAB) is the most commonly used diagnostic test to guide management of a patient with a thyroid nodule (s), it cannot distinguish FTC or HCC from their benign counterparts because capsular and vascular invasion cannot be assessed by FNAB. Thus, most FNAB results for follicular neoplasms fall into an indeterminate category, such as follicular lesion of undetermined significance or follicular neoplasm, and surgical excision to obtain a tissue diagnosis is indicated. The diagnostic criteria for follicular neoplasm on FNAB include the presence of abundant follicular cells in microfollicles in the absence of colloid material (Fig. 24.2a, b) (see Chap. 5).
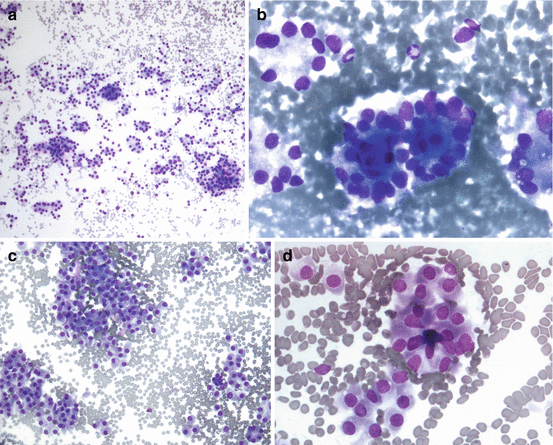

Fig. 24.2
Cytologic features of (a) follicular neoplasm (low power): cellular aspirate showing follicular cells in crowded clusters, pseudorosettes, tubule-like formations, and singly, in a background of red blood cells and no colloid present. Diff-Quik, 100×; (b) follicular neoplasm with microfollicle pseudorosettes: follicular cells with diffuse nuclear enlargement arranged in microfollicles and pseudorosettes in a background of red blood cells. Diff-Quik, 600×; (c) Hürthle cell neoplasm (low power): cellular aspirate showing Hurthle cells with diffuse nuclear enlargement arranged in crowded clusters, pseudorosettes, and singly, in a background of red blood cells and no colloid. Diff-Quik, 200×; (d) Hürthle cell neoplasm with microfollicle pseudorosettes: Hürthle cells with diffuse nuclear enlargement and abundant granular cytoplasm, arranged singly, in pseudorosettes and a microfollicle, in a background of red blood cells. Diff-Quik, 400× (Courtesy of Dr. Armando Filie, Laboratory of Pathology, National Cancer Institute, NIH)
Hürthle cells are thyroid follicular cells characterized by a large polygonal shape with an abundant eosinophilic, granular cytoplasm that is rich in mitochondria and a large nucleus with a prominent nucleolus. Hürthle cell changes are seen in various thyroid conditions, including nonneoplastic pathology such as Hashimoto’s thyroiditis, Graves’ disease, and nodular goiter [23].
Molecular Markers
Although significant progress has been made in the molecular diagnosis of thyroid nodules in recent years, none of the available modalities can accurately differentiate FTC from follicular adenoma. A gene expression classifier (GEC) uses an expression panel of messenger RNAs to distinguish benign from malignant lesions in patients with indeterminate nodules. Afirma (Veracyte, San Francisco, CA) is a commercially available test that uses a messenger expression panel of 142 genes. The test has high sensitivity and a negative predictive value of >90 % in indeterminate nodules (atypia or follicular lesion of undetermined significance and follicular or Hürthle cell neoplasm), but the specificity is only 50 %. Thus, approximately half of benign nodules with indeterminate cytology are falsely classified as “suspicious for malignancy” [25, 26].
Because the false-negative rate (5–8 %) of the Afirma test is similar to the rate of malignancy in patients with atypia or follicular lesion of undetermined significance (5–15 %), it remains unclear whether the test will alter a surgical decision. Li et al. conducted a cost-effective analysis and suggested that GEC testing may reduce the overall cost due to 74 % fewer surgeries for benign nodules, with no greater number of untreated cancers [27].
Point mutations in BRAF V600E and RAS (H-RAS, N-RAS, K-RAS) and RET/PTC and PAX8/PPARg gene arrangements have been identified in >70 % of thyroid cancer with high positive predictive values. The performance of previous-generation tests was not accurate enough to distinguish FTC or HCC from adenoma [28–30]. A recent study by Nikiforov et al. of 143 FNABs with a cytologic diagnosis of follicular neoplasm showed promising results in a comprehensive panel (ThyroSeq v2 NGS) that tests for point mutations in 13 genes and 42 types of gene fusions that occur in thyroid cancers. The authors reported 90 % sensitivity, 93 % specificity, and 92 % overall accuracy [31].
Pathological Diagnosis
The WHO defined FTC as “a malignant epithelial tumor showing follicular cell differentiation and lacking the diagnostic nuclear features of papillary thyroid carcinoma” [1]. Two main features in diagnosing FTC are evidence of capsular and or vascular invasion and the absence of the nuclear features of PTC in a tumor arising from follicular thyroid cells [17]. The combination of using strict diagnostic criteria for recognizing capsular and/or vascular invasion in the absence of the nuclear features of PTC and the trend toward an increase diagnosis of follicular variant of PTC has resulted in the increasing number of PTC diagnoses and a decrease in the percentage of FTCs among well-differentiated thyroid carcinomas [1, 17, 32].
Follicular adenoma commonly presents as a solitary lesion in an otherwise normal thyroid gland without evidence of invasive growth. Histologic features of adenomas include a microfollicular or macrofollicular growth pattern and lack degenerative changes, such as hemorrhage, fibrosis, and cyst formation [33] (Fig. 24.3a).
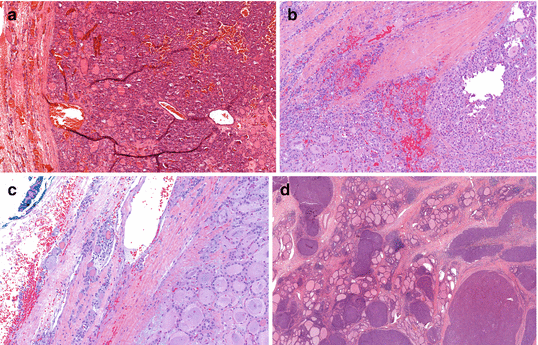
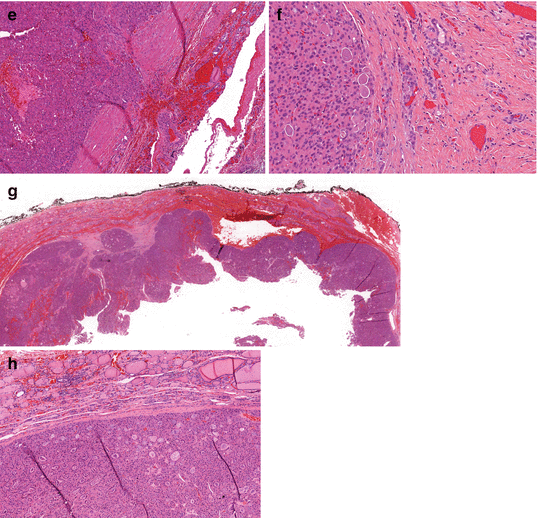


Fig. 24.3
Histologic features of (a) follicular adenoma: tumor with follicular cells, thick capsule, without any evidence of vascular or capsular invasion. Hematoxylin and eosin, 8×; (b) minimally invasive follicular thyroid carcinoma with capsular invasion. Hematoxylin and eosin, 12×; (c) minimally invasive follicular thyroid carcinoma with vascular invasion. Hematoxylin and eosin, 15×; (d) widely invasive follicular carcinoma with no complete capsule and extensive invasion. Hematoxylin and eosin, 2×; (e) minimally invasive Hürthle cell carcinoma with capsular invasion. Hematoxylin and eosin.10×; (f) minimally invasive Hürthle cell carcinoma with vascular invasion. Hematoxylin and eosin. 30×; (g) widely invasive Hürthle carcinoma. Hematoxylin and eosin. 0.7×; (h) Hürthle cell adenoma with no evidence of caspsular or vascular invasion. Hematoxylin and eosin. 8× (Courtesy of Drs. Drew Pratt and Martha Quezado, Laboratory of Pathology, National Cancer Institute, NIH)
Most FTCs are well-encapsulated solid tumors with a grayish tan to brown color at the cut surface [34]. MIFTCs typically have thicker and more irregular capsules than follicular adenomas, but are otherwise grossly indistinguishable. The presence of a capsule is an important feature that distinguishes MIFTC from WIFTC. WIFTCs may occur as partially encapsulated tumors with extensive penetration of the capsule or as multinodular, bulky tumors without a capsule, occasionally showing permeation of the thyroid blood vessels [34] (Fig. 24.3b–d). Multifocality in FTC is uncommon. The term MIFTC covers a wide range of pathology, from tumors with minimal capsular invasion only to those with extensive vascular invasion [16]. Vascular invasion is defined as the presence of tumor cells that are attached to the wall of capsular vessels [35]. Rosai has classified encapsulated tumors, or MIFTC, into three categories based on the presence and the extent of vascular invasion: (1) capsular invasion only, (2) limited vascular invasion (fewer than four sites), and (3) extensive vascular invasion (four or more sites) [36]. Others consider MIFTC as tumors with capsular invasion only [37, 38]. Because vascular invasion is an adverse prognostic feature that is associated with higher rates of recurrence and mortality [6, 39], it is suggested that MIFTC be categorized by the presence of vascular invasion, as MIFTC without vascular invasion has an excellent prognosis [40], as compared with MIFTC with vascular invasion [6]. In a large cohort of patients with FTC, Ito et al. demonstrated that extensive vascular invasion and tumor size >4 cm were independently associated with mortality in patients with MIFTC [41]. MIFTC with extensive vascular invasion was associated with higher recurrence and distant metastasis [42, 43].
Treatments
Surgical Management
A patient who has a cytologic diagnosis of follicular or Hürthle cell neoplasm should at least undergo a diagnostic thyroid lobectomy and isthmusectomy because the risk of malignancy is 15–30 % [45]. A total thyroidectomy should be considered in patients who are at risk for thyroid cancer, such as those who have had prior head/neck irradiation or a family history of thyroid cancer or those who have contralateral nodules, hypothyroidism, or clinical evidence of malignancy, such as signs of local invasion, suspicious sonogram findings, or lymphadenopathy.
Because the diagnosis of FTC and HCC is based on evidence of vascular or capsular invasion, intraoperative frozen section analysis of the thyroid nodule is inaccurate, as it is impractical to examine the entire capsule of the nodule and the histologic morphology of frozen tissue is inferior to that of formalin-fixed tissue because of the presence of frozen artifacts [46, 47].
However, intraoperative frozen section analysis is accurate in detecting lymph node metastasis. Once lymph node metastasis is confirmed, total thyroidectomy with a compartment-oriented lymph node dissection should be performed.
Published papers on MIFTC and HCC are limited and often lack statistical power due to the rarity of the disease. The classification of FTC and HCC by the degree of invasion stratifies patients into high-risk (WIFTC) and low-risk (MIFTC) groups. The optimal surgical management of patients with MIFTC remains controversial. The 2015 American Thyroid Association guidelines recommend either a thyroid lobectomy or a total thyroidectomy for low-risk PTC or FTC between 1 and 4 cm. in size without extrathyroidal extension and without evidence of lymph node metastasis [48]. However, several centers consider a thyroid lobectomy as an adequate procedure for MIFTC with capsular invasion only, without vascular invasion, with a tumor size <4 cm, no evidence of nodal or distant metastasis, and patient age less than 45 years old. Candidates for total thyroidectomy are those who are more than 45 years old, have a tumor size >4 cm, or have a presence of vascular invasion or evidence of nodal or distant metastasis [40, 49–51]. A large series of MIFTC from Japan demonstrated that age greater than 45 years was the only independent prognostic factor for survival, while tumor size >4 cm and distant metastasis were significant adverse prognostic factors in univariate analysis. Findings suggest that younger patients with MIFTC have a good prognosis despite having large tumors or distant metastases [52]. A series from the Mayo Clinic showed 10-year cause-specific mortality and distant metastases of 28 and 19 %, respectively, in patients with MIFTC and vascular invasion, while none of the patients with capsular invasion only developed distant metastases or had mortality [40]. Thus, a completion thyroidectomy should be considered for patients with MIFTC who are more than 45 years old, have a tumor size >4 cm, and have a presence of vascular invasion or evidence of nodal or distant metastasis. Patients with WIFTC should undergo a total thyroidectomy to remove macroscopic disease, if recognized intraoperatively, or a completion thyroidectomy. Because the rate of lymph node metastasis in patients with FTC is low, a prophylactic compartmental lymphadenectomy is not recommended [16].
Optimal surgical management of a thyroid nodule with cytologic diagnosis of Hürthle cell neoplasm has been controversial but should include at least a diagnostic thyroid lobectomy. The clinical factors associated with HCC include gender (male), age (older), tumor size (>4 cm), and prior childhood head and neck irradiation [53–58]. A total thyroidectomy may be considered in patients with these clinical factors. Any suspicious lymph nodes found intraoperatively should be submitted for frozen section analysis. A compartment-oriented lymphadenectomy should be performed if lymph node metastasis is confirmed. The role of prophylactic central compartment lymph node dissection for HCC is not known. However, it should be considered in patients with risk factors or who are suspected to have widely invasive HCC (WIHCC), as lymph node metastasis is not uncommon and many HCCs do not take up radioiodine (RAI). McDonald et al. used AMES (age, metastasis, extent, and size) risk stratification to identify high-risk patients and found that the extent of the operation was the strongest risk factor for recurrence. Thus, the authors advocate more aggressive surgery for patients with one or more AMES risk factors [56].
Because patients with minimally invasive HCC (MIHCC), defined as either HCC with a minimal capsular invasion only [57] or a focus of capsular or vascular invasion [58], have an excellent prognosis, a thyroid lobectomy and isthmusectomy may be adequate. Two studies, one by Stojadinovic et al. (n = 23) and another by Sanders and Silverman (n = 12), found that none of the patients with MIHCC who underwent a thyroid lobectomy developed recurrence or mortality after a median follow-up of 8 and 14 years, respectively [57, 58].
Postoperative Management
Thyroid-Stimulating Hormone (TSH) Suppression Therapy
The 2015 American Thyroid Association management guidelines for differentiated thyroid cancer recommended the use of 2009 guidelines with additional clinical features to stratify patients into three categories by the risk of recurrence. The initial assessment for the risk of recurrence should be continually modified during the follow-up because the risks of recurrence or mortality can change as the result of change in clinical course or treatments:
- 1.
Low-risk patients: (a) no local or distant metastasis; (b) all macroscopic tumor tissue is removed; (c) no tumor invasion of locoregional tissues; (d) no aggressive histology or vascular invasion; (e) no RAI uptake outside the thyroid bed on the first whole-body scan; (f) clinical N0 or ≤5 pathologic N1 micrometastasis (<0.2 cm. in greatest dimension); (g) intrathyroidal FTC with capsular invasion and no or minimal (<4) foci of vascular invasion; (h) intrathyroidal papillary microcarcinoma including BRAF V600E mutation.
- 2.
Intermediate-risk patients: (a) microscopic tumor invasion into the perithyroidal soft tissues found at initial surgery; (b) 131I uptake outside the thyroid bed on the RAI whole-body scan performed after thyroid remnant ablation; (c) tumor with aggressive histology or vascular invasion; (d) clinical N1 or >5 pathological N1 with all involved lymph nodes <3 cm in greatest dimension; (e) multifocal papillary microcarcinoma with extrathyroidal extension and BRAF V600E mutation.
- 3.
High-risk patients: (a) macroscopic tumor invasion; (b) incomplete tumor resection; (c) distant metastases; (d) thyroglobulinemia out of proportion to what is seen on the posttreatment scan; (e) pathologic N1 with any metastatic lymph node >3 cm. in greatest dimension; (f) FTC with extensive (≥4 foci) vascular invasion.
The initial dose of levothyroxine in patients with thyroid cancer is 2 μg/kg. Patients with excellent response, defined as non-stimulated Tg < 0.2 ng/ml or TSH-stimulated Tg < 1 ng/ml with no radiographic evidence of tumor, do not require TSH suppression. TSH should be maintained at 0.5–2.0 mU/L. If non-stimulated Tg ≥ 0.2 ng/ml in low-risk patients (indeterminate or incomplete response), TSH should be maintained at 0.1–0.5 mU/L. Initial TSH goal for intermediate-risk and high-risk patients should be at 0.1–0.5 mU/L and <0.1 mU/L, respectively [48]. Thyroid function tests should be monitored every 6–8 weeks after initial administration of levothyroxine until no adjustment is needed.
Radioactive Iodine Treatment
RAI is the most effective treatment option for microscopic foci of distant metastatic differentiated thyroid carcinoma. An additional benefit of using RAI is that it facilitates the use of serum Tg as a marker for recurrent or persistent disease in the absence of thyroid tissue. RAI is not routinely recommended in low-risk patients. RAI should be considered following a total thyroidectomy or a completion thyroidectomy in patients with WIFTC or WIHCC, who are more than 45 years old and have a tumor size >4 cm. RAI is recommended as it improves disease-specific and disease-free survival in patients who have extensive vascular invasion or gross extrathyroidal invasion or evidence of distant metastasis [16, 48, 52].
Because MIFTC has an excellent prognosis, there is no evidence that the use of RAI following a total thyroidectomy improves patient outcome. A population-based study of survival among 1200 patients with MIFTC suggested that the use of RAI following total thyroidectomy did not improve patient survival [11].
In contrast to other differentiated cancers of follicular cell origin, HCCs are less RAI avid. Less than 10 % of HCC concentrate iodine in patients with known distant metastasis [12]. However, postoperative RAI ablation should still be performed to facilitate surveillance using serum Tg. The revised ATA guidelines recommend 100–200 mCi and 30–100 mCi 131I for patients with known or suspected residual disease and those without, respectively, followed by post-therapy whole-body scan [59].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree