EXAMPLES OF FEEDBACK IN ENDOCRINE SYSTEMS
Importantly, our current state of knowledge does not allow us to apply all these principles to most endocrine systems. Nonetheless, there is plentiful evidence for the existence of feedback regulation in all endocrine systems. Knowledge of this feed-back has been used in the design and interpretation of many clinical tests for the determination of endocrine disorders.
HYPOTHALAMIC–PITUITARY–TARGET ENDOCRINE FEEDBACK
LONG-LOOP FEEDBACK
One of the most clinically obvious and simplest forms of negative feedback control in endocrine systems involves suppression of the secretion of a trophic factor (or hormone) by the hormone it stimulates. For example, hormone A stimulates the secretion of hormone B, which in turn suppresses the secretion of hormone A. Hormone B may suppress the secretion of hormone A by acting directly on the cells that secrete A, or indirectly, by acting on the cells (or neurons) that stimulate the secretion of A. This type of control is exemplified in the relations between the hypothalamus, anterior pituitary gland, and peripheral endocrine glands controlled by pituitary hormones. The hypothalamus secretes neurohormones that stimulate (or inhibit) the secretion of specific anterior pituitary hormones, which in turn stimulate a peripheral target gland to secrete hormone and, with sufficient stimulation, to grow (see Chap. 8 and Chap. 9).
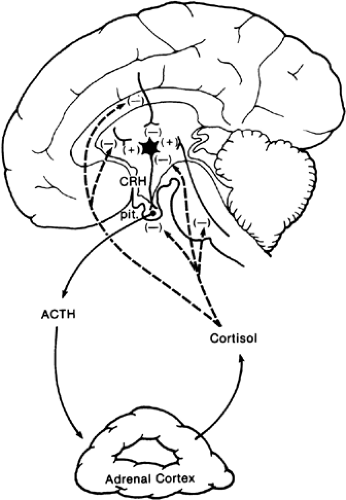
In Figure 5-4, CRH-containing neurons in the hypothalamus release CRH into the hypophysial portal system.3 ACTH released from corticotropes in response to CRH stimulates cortisol synthesis and secretion from the adrenal cortex. Cortisol acts to inhibit the secretion of ACTH from the corticotrope and to inhibit CRH secretion from the hypothalamic neuron, and it may also act on extrahypothalamic sites that regulate CRH synthesis and secretion.4,5 Clinically, the feedback effects of cortisol are important. Long-term therapy with pharmacologic amounts of glucocorticoids suppresses ACTH secretion to the extent that the adrenal cortices atrophy and become unresponsive to ACTH. The atrophic adrenal cortex does not secrete normal quantities of cortisol, and the abrupt discontinuation of exogenous glucocorticoids may lead to a patient who displays all the signs of cortisol deficiency (see Chap. 76 and Chap. 78).
The feedback effects of cortisol on ACTH release is an example of long-loop feedback: secretion of the peripheral gland affecting the secretion of the pituitary trophic hormone. Long-loop feedback occurs in most of the anterior pituitary hormone systems and is most apparent when the capacity for hormone synthesis or secretion in the peripheral target gland is compromised or abolished. The magnitude of the effects of inhibiting or removing the long-loop feedback signal on circulating concentrations of the appropriate pituitary trophic hormone (i.e., the effects of opening the feedback loop) is large. The dramatic increases in the circulating concentrations of the pituitary trophic hormone that occur under conditions in which there is an abnormally low feedback signal from the target endocrine gland are useful clinically in distinguishing between a primary and a secondary disturbance in an endocrine system. For instance, hypothyroidism could arise from a primary disturbance in the synthesis of thyroxine in the thyroid gland, or it could result from lack of stimulation of the thyroid gland by thyrotropin (thyroid-stimulating hormone [TSH]). In both cases, circulating thyroxine concentrations would be low; if the defect were due to a primary thyroidal disturbance, TSH concentrations would be high, whereas if the defect were due to lack of TSH secretion, circulating concentrations would be low.
SHORT-LOOP FEEDBACK
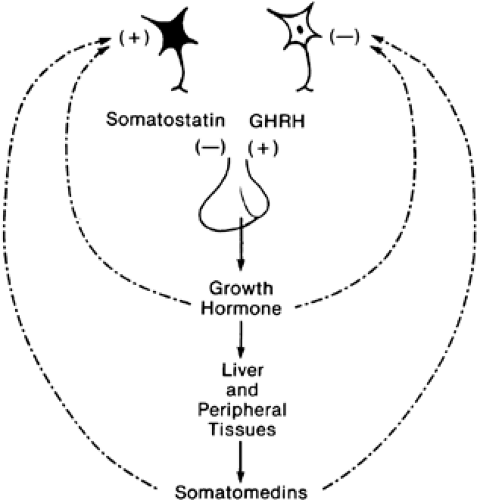
Evidence also exists for short-loop inhibition of secretion of hypothalamic-releasing hormones by the trophic hormones of the anterior pituitary. For example, growth hormone secreted by somatotropes in the anterior pituitary stimulates secretion of insulin-like growth factors from the liver and other peripheral tissues (see Chap. 12 and Chap. 173). The somatomedins exhibit long-loop feedback on growth hormone secretion. However, growth hormone has an effect on the hypothalamic-releasing and release-inhibiting hormones that regulate its secretion (Fig. 5-5). There is evidence suggesting that this feedback can occur either by inhibiting the secretion of growth hormone-releasing hormone or by stimulating the secretion of growth hormone-inhibiting hormone (somatostatin) into the hypophysial portal circulation.6,7 There is growing evidence that similar short-loop feedback circuits exist for the other hypophysial hormones. Quantitatively, these appear to be less important than the long-loop feedback, and they may serve as a fine-tuning system within the central nervous system.
ULTRASHORT-LOOP FEEDBACK
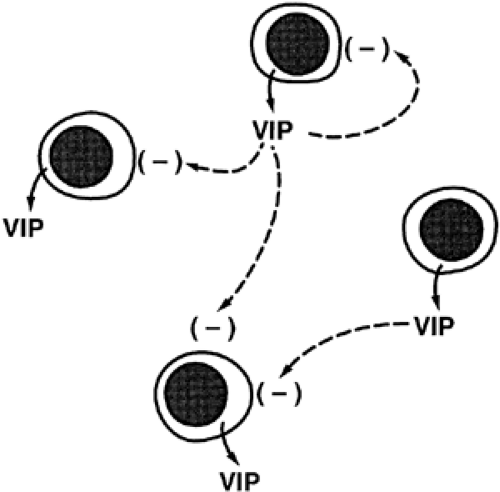
Ultrashort-loop feedback is suspected to occur when a hormone acts on its own cell type to inhibit further secretion of itself. There is little evidence for this phenomenon; however, it could be of considerable importance in the regulation of secretion of posterior pituitary hormones. In the hypothalamus, collateral axons of oxytocin and vasopressin neurons make contact with other oxytocin and vasopressin neurons.8,9 These cell–cell interactions could help to sharpen and synchronize the secretion of hormone in a given cell type. In the anterior pituitary, vasoactive intestinal peptide (VIP) stimulates secretion of prolactin from lactotropes. However, this effect may be autocrine/paracrine in nature, because lacto-tropes express and secrete both VIP and prolactin into the extra-cellular fluid (Fig. 5-6). Thus, VIP can then stimulate secretion of VIP/prolactin from its own or neighboring lactotropes.10,11 and 12
FEEDBACK EFFECTS ON AMPLITUDE AND PULSE FREQUENCY: THE GONADOTROPIN SYSTEM
Many, and perhaps most, anterior pituitary hormones are secreted in a pulsatile manner (i.e., surges in secretion occur at regular intervals). Evidence suggests that this arises from synchronous bursts of activity in hypothalamic neurons that secrete the appropriate releasing factors. The gonadal endocrine system has been best studied from this point of view. The gonadotropin-releasing hormone (GnRH) is released in bursts that appear to drive secretion of luteinizing hormone (LH) in episodes (see Chap. 16). In humans and in subhuman primates, GnRH and LH concentrations in hypophysial and systemic blood demonstrate cycles with periods of 2 to 4 hours. LH stimulates testosterone secretion in men and ovulation, luteal formation, and estrogen and progesterone secretion in women.
NEGATIVE FEEDBACK BY SEX STEROIDS
Testosterone, estrogen, and progesterone exert long-loop feed-back on the cycle amplitude and frequency of GnRH and LH. Testosterone and progesterone decrease the frequency and amplitude of the GnRH cycles and thus lower the mean concentration of LH in the circulation. The absence of both hormones leads to acceleration of the cycle frequency and amplitude. The effects of estradiol are complex. Low concentrations of estradiol or high concentrations of estradiol in the presence of progesterone inhibit both the frequency and amplitude of LH secretory pulses, thus decreasing both minimum and maximum concentrations of LH in the circulation.13,13a,14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree