Chapter 1 Establishing the Diagnosis of HIV Infection
Treatment, especially highly active antiretroviral therapy (HAART), has dramatically improved survival for persons infected with human immunodeficiency virus (HIV).1 However, because of late diagnosis, many people do not realize the full benefits of therapy. In 2004, 39% were diagnosed with acquired immunodeficiency syndrome (AIDS) within 1 year of their first positive HIV test.2 Although many with HIV infection visit healthcare settings in the years before their diagnosis, they are often not tested for HIV.3–5 Moreover, the changing demographics of the HIV/AIDS epidemic in the US have diminished the effectiveness of testing based solely on reported risk behaviors to identify HIV-infected persons.6 Since the 1980s, increasing proportions of infected persons are found among youth, minority races and ethnicities, persons who reside outside metropolitan areas, and heterosexual men and women.
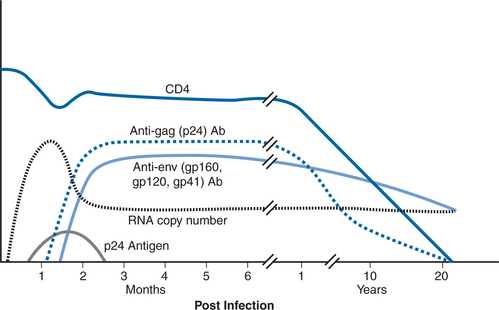
During its clinical stages, HIV infection produces numerous biologic indicators of virus infection including clinical symptoms, viremia, circulating viral proteins, antibodies against viral proteins, and immunologic markers of infection such as changes in the absolute number of and ratio of CD4 and CD8 lymphocytes (Fig. 1-1). These markers can be detected and in many cases quantified by the correct use of diagnostic tests to substantiate the presence of HIV infection. Selecting the most appropriate test for accurate diagnosis depends upon knowledge of the kinetic characteristics and relationships of the various biologic markers and the performance characteristics (sensitivity and specificity) of the various detection systems. This chapter reviews various techniques currently used in the diagnosis of HIV infection (Table 1-1) and their sequence of reactivity over the course of HIV infection.
Table 1-1 Tests Available for HIV Diagnosis
| Antibody Blood Tests |
| EIA for antibody to HIV-1, HIV-2, HIV-½ (serum/plasma) Rapid antibody tests for HIV-1, HIV-½ (serum/plasma/whole blood) Indirect IFA for HIV-1 (serum/plasma) Western blot for HIV-1, HIV-2 (serum/plasma) |
| Other Blood Tests |
| HIV-1 p24 antigen EIA (serum/plasma) Combination HIV-1 p24 antigen–HIV-½ antibody assays (serum/plasma) HIV-1 DNA PCR (PBMCs) Qualitative HIV-1 RNA RT-PCR assays (plasma) Qualitative HIV-1 RNA TMA assays (plasma) Cell culture for HIV-½ (plasma/peripheral blood mononuclear cells) |
| Other Fluid Tests |
| Oral fluid HIV-1 antibody EIA/Western blot Oral fluid HIV-½ rapid antibody test Urine HIV-1 antibody EIA/Western blot |
| Quantitative HIV-1 RNA Viral Load (Plasma) |
| RT-PCR Branched bDNA NASBA |
THE HIV VIRION
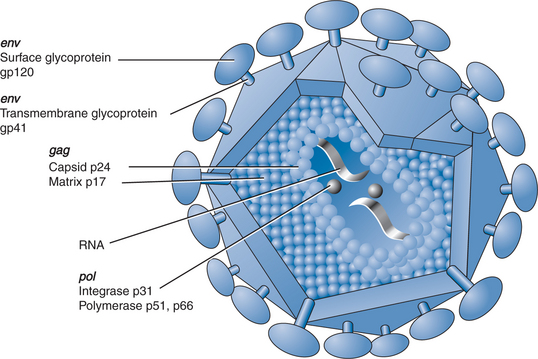
Morphologically, HIV is an enveloped, roughly spherical virus that contains a capsid enclosing two copies of genomic RNA (Fig. 1-2).7 Replication, via the action of the enzyme reverse transcriptase, proceeds by the reverse transcription of genomic RNA into a DNA intermediate. The DNA intermediate is integrated into the host cell genome where it is referred to as proviral DNA. Provirus is the template from which progeny RNA and proteins are synthesized using the host cell’s synthetic machinery. The viral components subsequently assemble below the host cell membrane and bud from the membrane as mature virions potentially capable of spreading infection to other cells or transmitting infection to other hosts.8 HIV replicates in an error-prone manner that generates a mutation virtually every time it replicates.9 Thus, the viruses circulating in the host are not an absolutely homogeneous population; rather, they consist of a swarm of similar yet divergent variants known as quasi-species. This existing variation and ongoing mutation is the substrate upon which selective pressures act to allow the emergence of different variants in the host such as drug resistant strains or immunologic escape-mutants.
The HIV genome consists of three major genes that encode the viral enzymatic and structural proteins: gag (group-specific antigens or capsid proteins), pol (polymerase, protease, and integrase enzymes), and env (envelope glycoproteins).10
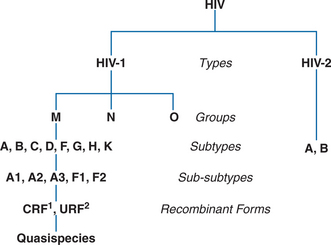
HIV viruses are classified based on phylogenetic relatedness of nucleotide sequences. The current classification is hierarchical and consists of types, groups, subtypes, sub-subtypes, and recombinant forms (Fig. 1-3).11 Subtypes are often referred to as clades. The most distantly related HIVs are categorized as types: HIV type 1 and HIV type 2.12 The HIV-1 type is further characterized into groups: the major (M) group; the more divergent outlier (O) group, and the non-M, non-O (N) group.9,12 Most HIV infections occur with group M HIV-1 which is differentiated into subtypes (HIV-1 group M subtypes A, B, C, D, F, G, H, J, and K).9 Subtypes A and F are further classified into sub-subtypes A1, A2, A3, F1, and F2. HIV-2 has two major subtypes, A and B.12 When viruses from two or more HIV-1 lineages infect one individual and exchange their genetic material, they are termed recombinant viruses.13 If transmission of the recombinant virus has been documented by whole genome sequences to three or more persons, it is referred to as a circulating recombinant form (CRF), and the CRF is given a numeric designation. As of July 2007, CRF01 to CRF34 are recognized. Recombinant viruses that have been identified but are not documented to have been further transmitted are referred to as unique recombinant forms (URF). The variation in nucleotide sequences can have implications for the biology and transmission of the virus, for patient survival, and also helps explain the geographic distribution, and epidemiology of HIV infection.9,12,13 From the diagnostic perspective, sequence variation can have significant implications for the reactivity and cross-reactivity of diagnostic tests designed to detect specific viral proteins or peptides.
NATURAL HISTORY AND RESPONSETO HIV INFECTION
Clinically, HIV infection progresses through several stages, each with characteristic laboratory findings. Within weeks of infection, a flu-like syndrome may develop in some 40–90% of patients.14–16 Symptoms, including fever, malaise, myalgia, lymphadenopathy, and macular rash, lasting from a few days to several weeks, can be sufficiently severe to prompt as many as half of patients to seek medical attention.16 Primary HIV infection or PHI, as the syndrome is called, lasts a few weeks and is associated with high levels of viremia, a transient depression in CD4 T lymphocytes, elevation of CD8 T lymphocytes, and generally resolves concomitantly with the onset of a cellular and humoral immune response to HIV. Following PHI, most patients then enter an asymptomatic or “latent” phase that has been observed to last from several months to more than 15 years. During this clinically latent phase, HIV replication establishes a steady state where virus levels in plasma reach a “set point” and CD4 T lymphocyte counts slowly decline. Both the viral set point and rate of decline of CD4 T-lymphocyte levels have prognostic value in predicting progression to advanced disease.17 With continued viral replication and progressive decline in CD4 T lymphocytes, patients may develop constitutional symptoms (fever, malaise, lymphadenopathy) and non-life-threatening diseases related to immunodeficiency such as oral candidiasis, oral hairy leukoplakia, and thrombocytopenia. Finally, and often precipitously, CD4 T-lymphocyte levels decline to less than 200 cells/μL, virus levels climb, the immune system collapses, and patients are prone to life-threatening opportunistic infections and malignancies–the full-fledged AIDS.
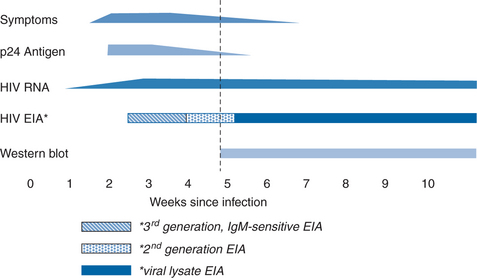
There are corresponding phases in reactivity of various laboratory diagnostic tests during clinical stages of HIV infection (Fig. 1-4). Shortly after infection, viral replication reaches levels that can be detected by sensitive molecular assays for viral nucleic acid (nucleic acid amplification tests or NAATs). The duration of time between initial infection and first detection of viral nucleic acid in plasma, the “eclipse period”, has not been accurately defined and likely varies with the infection route and inoculum size as well as with the sensitivity of the particular NAAT assay. The eclipse period has been estimated by back regression of the initial slopes of a plot of virus load versus time and by analysis of patients with an accurately known infection event. These types of studies estimate a duration of ∼6–12 days for the eclipse period.18,19 Viral antigens, particularly the most abundant viral protein, the capsid protein p24, are also detectable in the first few weeks after infection. There are ∼2000–3000 copies of the p24 molecule in each virion, compared with two RNA molecules. Detection thresholds for p24 assays during acute infection correspond to ∼10 000 viral RNA copies per milliliter; p24 antigen can first be detected ∼5 days after plasma viral RNA.18,19 p24 antigen detection is transient and its disappearance usually coincides with the development of anti-p24 antibodies, whereas RNA usually remains detectable throughout infection. The disappearance of p24 antigen with seroconversion may be due in part to blocking of p24 detection by the formation of immune complexes with anti-p24 antibody. The reappearance of detectable p24 antigen in late-stage disease is associated with the reduction in p24 antibody and the increased viral replication that occurs in that phase.
Specific anti-HIV antibody signifies past exposure to HIV and current active infection. Patients mount and generally sustain a vigorous humoral immune response to HIV infection. Detection of anti-HIV antibodies is the mainstay of diagnosis for HIV infection in the vast majority of patients. Immunoglobulin M (IgM) antibodies to HIV appear first. They are of low titer, usually transient, but may be detected intermittently throughout infection.20–23 The IgM response is followed by a long-lived immunoglobulin G (IgG) response. Antibodies to protein products of the gag gene (p17, p24, and p55) and env gene (gp41, gp120, and gp160) appear first, followed days to weeks later by antibodies to pol gene proteins (p31, p51, and p66).18,19,24 The period of time from infection until antibody seroconversion (known as the preseroconversion window period) varies between different persons and also depends on the assay used to detect antibody (Fig. 1-4). The newer, most sensitive third generation enzyme immunoassays (EIAs) are generally positive 7–14 days after viral RNA is first detected.18,19 It may take longer (15–30 days) for earlier, less sensitive first generation EIAs to become reactive, or for development of a sufficient number of bands on Western blot to meet diagnostic criteria.18,19 Almost all patients meet the criteria for serologic diagnosis of HIV infection within 3 months after infection.18,19,24–27 In cases in which either the EIA or Western blot assays are found to be negative or indeterminate, repeat testing is recommended 8–12 weeks later. In rare cases, infection can be documented by virologic techniques, but seroconversion is delayed or absent.28–30 Usually, such cases have extremely high levels of viremia and rapid progression of clinical disease. It is not clear why this occurs. Some patients have an underlying, preexistent immunodeficiency such as common variable immunodeficiency, but most do not.19,25,26,31–34 Perhaps it is due to B-lymphocyte loss or dysfunction, although most of these patients appear to generate antibodies against other pathogens.30 With advanced disease or with effective antiretroviral therapy in patients with established seropositivity, antibody levels decline, but total loss of seroreactivity is extremely rare.31,34–37 Early and intensive antiretroviral therapy, initiated before seroconversion, can also blunt or abolish the antibody response to HIV infection.31,35,38 Spontaneous seroreversion, in the absence of early therapy or advanced disease, is extremely rare and has been difficult to document.39,40
METHODOLOGIES
Screening and Confirmatory Tests for Diagnosis of HIV Infection
A listing of HIV diagnostic tests approved by the US Food and Drug Administration (FDA) is at http://www.FDA.gov/cber/products/testkits.htm. The World Health Organization (WHO) also evaluates HIV tests and maintains a list of HIV tests and results of their evaluation at http://www.who.int/diagnostics_laboratory/publications/evaluations/en/index.html.
EIA for Detection of HIV Antibody
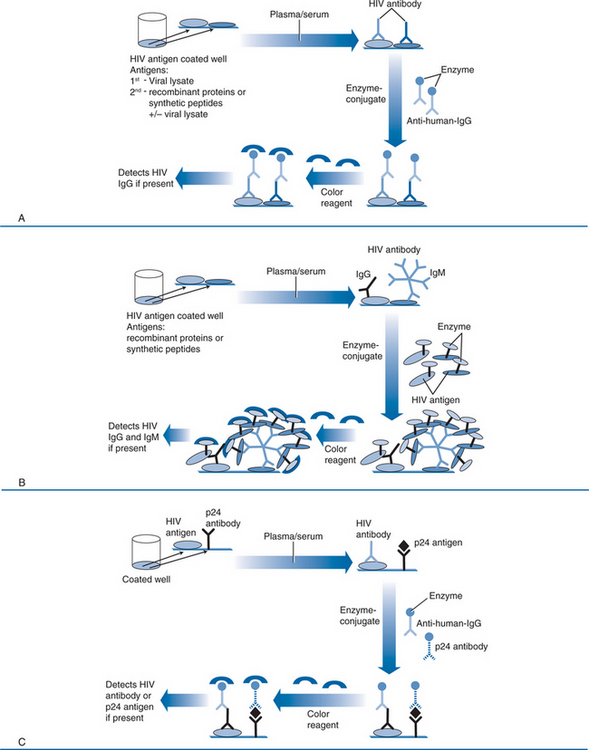
In the earliest first generation versions of the EIA, antigen in the form of a whole-virus lysate is coated on the surface of microtiter wells as the solid-phase antigen (Fig. 1-5A). The HIV-1 viral lysates contain native antigens from virtually all structural components of the virus, and will generally detect all subtypes of group M, many group O, and cross-react with many HIV-2 viruses. However, they are prone to false-positive results because of cross-reactions with host cell proteins from which the virus is propagated, and they are less sensitive during early seroconversion because they do not detect IgM and because of the large dilutions necessary to attain adequate specificity. The second generation tests replace crude viral lysates with recombinant viral proteins or peptides from conserved immunodominant regions of HIV-1 and HIV-2 (Fig. 1-5A), and many have been modified to detect the outlier (O) group of HIV-1. Not only is the sensitivity for detection of a range of HIV types and subtypes expanded, but the false-positive rate due to reaction with viral lysate contaminants or cross-reactions with viral antigens is lower, resulting in greater specificity. Third generation tests (or immunometric tests) rely on a somewhat different principle (Fig. 1-5B). Like first and second generation tests, viral antigens are in the solid phase. In third generation tests, however, detection is accomplished with enzyme conjugated to HIV antigen (instead of to anti-human IgG). Antibody in patient serum thus binds to HIV antigen in the solid phase and also to the HIV antigen in the enzyme conjugate. This “antigen sandwich” detects any class of antibody, including IgM, and is subject to lower background ODs, which improves analytic sensitivity. As a result, the third generation tests detect seroconversion about a week before second generation tests.18,19,41,42 Newer third generation assays incorporate antigens from HIV-1 group M subtypes, group O and HIV-2. These combination formats have excellent sensitivity when assessing subjects with established infection and may have lower false-positive rates (i.e., higher specificity) than earlier generation tests.18,19,41,43–48 Fourth generation EIAs have been developed that combine antibody and p24 antigen detection in the same assay (Fig. 1-5C).47,49,50 This increases sensitivity during acute infection, because antigen detection has been shown to shorten the preseroconversion window by ∼5 days. However, the fourth generation assay requires a more formidable confirmatory procedure to validate reactivity against antibody or antigen. Further, the combination assay may not be as sensitive for antigen detection as separate p24 antigen testing, and the test may not be equally sensitive for all subtypes of HIV-1.51
All EIAs are subject to false-positive reactions. These may be due to procedural mistakes, coincidental cross-reactions, or be associated with acute or chronic inflammation, autoimmunity, or hypergammaglobulinemia (Table 1-2).52–54 False positives related to the latter associations are usually transient or unstable and do not confirm with supplemental tests based on a different principle. HIV vaccine-induced antibodies may also be detected by current tests and may cause false-positive results in vaccine trial participants; true infection status can be difficult to resolve. Persons whose test results are HIV-positive and who are identified as vaccine trial participants should be encouraged to contact or return to their trial site or an associated trial site for confirmatory testing. A referenced list of possible and variably documented associations with false-positive HIV EIA results can be found at http://healtoronto.com/testcross.html.
Table 1-2 Causes of False-Positive or False-Negative HIV EIA Results
| Causes of False-Positive Results for HIV Antibody on EIA |
| Hematologic malignant disorders DNA viral infections Autoimmune disorders Multiple myeloma Primary biliary cirrhosis Alcoholic hepatitis Influenza vaccination Hepatitis B vaccination Rabies vaccination Passively transferred antibodies Antibodies to class II leukocytes Renal transplantation Chronic renal failure Stevens–Johnson syndrome Positive rapid plasma regain test Malaria Dengue Increased parity Systemic lupus erythematosus |
| Causes of False-Negative Results for HIV Antibody on EIA |
| Preseroconversion “window’ period Immunosuppressive therapy Replacement transfusion Malignant disorders B-lymphocyte dysfunction Bone marrow transplantation Starch powder from laboratory gloves |
Western Blot
The HIV Western blot identifies antibodies to individual viral proteins. Because the technique separates and concentrates specific viral proteins for detection and identification of specific anti-HIV antibodies, it is generally considered to be a more specific test than the EIA. The Western blot is widely used to confirm HIV infection in specimens that have screened as positive by EIA. To create the Western blot, HIV proteins from a viral lysate are subjected to gel electrophoresis, separating the component viral proteins based on their molecular weights. Higher molecular weight proteins have slower mobility than lower molecular weight proteins. The individual proteins are then transferred as resolved proteins to nitrocellulose paper. When the strips are reacted with test sera, antibody to the viral proteins binds at the location where the HIV protein is immobilized. Bound antibody is then detected with anti-IgG enzyme conjugated to a colorimetric substrate. This produces a colored band corresponding to the particular viral protein’s molecular weight (Fig. 1-6).
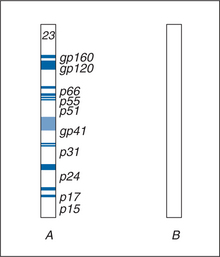
Figure 1-6 Western blot results: (A) positive result (reactive to all antigens); (B) negative result.
Reprinted with permission from Contantine NT, Callahan JD, Watts DM. Retroviral Testing: Essentials of Quality Control and Laboratory Diagnosis. Boca Raton, FL: CRC Press; 1992, p 63.
The nomenclature of viral proteins indicates either “gp” for glycoprotein or “p” for protein followed by a number indicating its molecular weight in kilodaltons. The glycoprotein bands on Western blot tend to be diffuse because of variable glycosylation, whereas the protein bands are more discreet. The major bands of diagnostic utility for HIV-1 include envelope proteins (gp160, gp120, gp41), the gag core gene proteins (p55, p24, p17), and polymerase gene proteins (p66, p51, p31). HIV-2 Western blot bands are similar but differ somewhat in the molecular weight of the three gene products (Table 1-3). Other bands corresponding to smaller viral proteins may be present but are not part of the diagnostic scoring criteria.55
Table 1-3 HIV Proteins Represented on Western Blot Test
| Viral Protein/Glycoprotein Molecular Weight | ||
|---|---|---|
| HIV Gene and Products | HIV-1 | HIV-2 |
| env | ||
| Precursor | gp160 | gp140 |
| External glycoprotein | gp120 | gp105/125 |
| Transmembrane glycoprotein | gp41 | gp36 |
| pol | ||
| Reverse transcriptase | p66 | p68 |
| Reverse transcriptase | p51 | p53 |
| Endonuclease | p31 | p31/34 |
| gag | ||
| Precursor | p55 | p56 |
| Core | p24 | p26 |
| Matrix | p17 | p16 |
The antigen preparation for Western blot is generally purified extracellular virus representing mature virions. The protein profile of mature virions as detected by Western blot may differ somewhat from that of intracellular replicating virus because not all proteins required for virus replication are packaged with extracellular virus. For example, gp160 is proteolytically cleaved by host cell proteases into gp120 and gp41. Intact gp160 is not efficiently incorporated into viral membranes, and its presence in extracellular virus is somewhat unexpected. Thus, only a small portion of the gp160 band in Western blot is authentic gp160; the majority represents polymerized gp41 (tetramers).56–59 Immunoblot assays that use purified recombinant proteins as an alternative to viral lysate antigens have also been developed. These perform as well as standard Western blot, though none are approved by FDA.60,61
Interpretation of the Western blot is somewhat subjective, and test specimens are compared to a control strip that has been reacted with low-positive serum. If no colored bands are present, the Western blot is interpreted as negative. Interpretive criteria for a positive Western blot place heavy emphasis on the presence of envelope glycoproteins and the core protein p24 because these appear first in serum following seroconversion (Fig. 1-1). Depending on which organization’s criteria are used, a blot is interpreted as positive when at least two bands or band clusters (e.g., p24, gp41, gp120/160) are present, or if one band from each of the gene products is present (Table 1-4).62–64 If bands are present but do not meet interpretive criteria for a positive test, the blot is scored as indeterminate.
Table 1-4 Interpretive Criteria for a Positive HIV-1 Western Blot Test
| Organization | Criteria |
|---|---|
| APHL/CDC | Any two: p24, gp41, gp120/160 |
| ARC | Three or more bands, one from each gene product group: gag, pol, env |
| CRSS | Two or more bands: p24 or p31 and gp41 or gp120/160 |
| WHO | Two env bands with or without gag or pol |
APHL/CDC, Association of Public Health Laboratories/Centers for Disease Control and Prevention; ARC, American Red Cross; CRSS, Consortium for Retrovirus Serological Standardization; WHO, World Health Organization.
Data from Proffitt MR, Yen-Lieberman B. Laboratory diagnosis of human immunodeficiency virus infection. Infect Dis Clin North Am 7:203, 1993; George JR, Schochetman G. Serologic tests for the detection of human immunodeficiency virus infection. In: George JR, Schochetman G (eds) AIDS Testing: Methodology and Management Issues. New York: Springer; 1992, p 48.
Given sufficient time after infection, most subjects develop a full banding pattern, and there is little difference in interpretation by the various scoring criteria.18,19,52,63,64 An indeterminate result may represent early seroconversion or reflect nonspecific, irrelevant cross-reactivity. In studies of serial specimens from early infection, the Western blot becomes criterion-positive 0–20 days after the EIA is positive (depending on the sensitivity of the EIA) and 10–30 days after tests for viral nucleic acid or antigen are positive.18,19,25,27,47,41 Viral nucleic acid or antigen testing may be a useful supplementary test for resolving indeterminate Western blot results.52,65–67 However, the ultimate resolution requires follow-up testing. Specimens from infected persons with an initially indeterminate blot will usually evolve into a pattern indicative of HIV infection within 4 weeks.18,19,25,27,41,47,67 Nonspecific indeterminate blot patterns generally do not evolve and may recede.67 The most common cause of an indeterminate Western blot result, which may occur in 10–15% of uninfected persons, is an isolated p24 or p17 band.47,53,63 In a long-term study of uninfected patients with repeatedly reactive HIV EIAs and indeterminate Western blots, independent risk factors for an indeterminate blot among males were a tetanus booster during the preceding 2 years and sexual contact with a prostitute. For uninfected women, indeterminate Western blots were associated with having given birth to one or more children, and the presence of rheumatoid factor or antinuclear antibodies.63 Other variably documented associations with nonspecific or indeterminate banding patterns are listed in Table 1-5.32,52,53
Table 1-5 Causes of False-Positive and Indeterminate Western Blot Results for HIV
| Normal human ribonucleoproteins Other human retroviruses Antibodies to mitochondrial, nuclear, and T-lymphocyte antigens Globulins produced during polyclonal gammopathy Proteins on filter paper Anticarbohydrate antibodies Heat-inactivated serum High concentration of bilirubin in serum Passively acquired antibodies Heterophil antibodies to bovine, ovine, caprine, and murine IgG HIV vaccines |
From Cordes RI, Ryan ME. Pitfalls in HIV testing: application and limitations of current tests. Postgrad Med 98:177, 1995; Willman JH, Hill HR, Martins TB, et al. Multiplex analysis of heterophile antibodies in patients with indeterminate HIV immunoassay results. Am J Clin Pathol 115:764, 2001.
False-positive Western blots are rare but problematic. Historically, Western blot positivity has become the gold standard for evaluating the sensitivity and specificity of other HIV tests. Discordance between a positive Western blot result and an independent test is generally ascribed to imperfect sensitivity of the independent test rather than to the imperfect specificity of the blot.52,65,66 Kleinman et al52 evaluated blot performance in a blood donor population, a low prevalence setting where the proportion of false positives is expected to be magnified. Specimens with criterion-positive blots were subjected to supplemental testing and follow-up to assign true infection status. Of the positive blots, 4.8% were thought to be false positive. A true positive was more likely in first time donors, when a p31 band was present, or when three or more bands were present. The probability that a positive blot is a true positive (positive predictive value, PPV) varies with prevalence. In this study with an HIV prevalence of 0.008%, PPV was 95%. In populations with prevalence above 0.1%, the PPV of a positive blot exceeds 99.9%.
Indirect Immunofluorescence Assay
The indirect immunofluorescence assay (IFA) is an alternative supplemental test used following a positive EIA result. In the IFA, HIV-infected cells are fixed on a microscope slide. The cell preparations are incubated with patient serum, washed, and further incubated with a fluorescein-labeled anti-human IgG reagent. Uninfected cells serve as an internal controlfor nonspecific binding or reaction with cellular antigens. Antibody-positive sera exhibit a characteristic cytoplasmic fluorescence. If both the control cells and virus-infected cells are fluorescent, the IFA is reported as indeterminate. Results are generally concordant with that of the Western blot.68 The IFA is sometimes used to resolve the infection status of a patient with an indeterminate Western blot. Although turnaround time is similar to that for the Western blot, the IFA is not widely used because it requires more hands-on time and a technician who is skilled at reading the IFA.
Simple, Rapid HIV Antibody Tests
A major advance has been the development of rapid HIV assays that provide results within an hour of sample collection without the need for specialized equipment or sophisticated technical expertise. A number of rapid tests can be performed on whole blood and some on oral fluid, in addition to serum or plasma. These features greatly enhance opportunities for HIV testing in resource-limited and outreach settings. Programmatic studies have indicated that the use of on-site rapid tests significantly increases the number of clients who actually receive their test results compared with conventional EIA testing.69–73 Rapid HIV tests also have demonstrated value for clinical situations in which decisions regarding antiviral prophylaxis are urgent, such as for pregnant women who present in labor with unknown HIV infection status, and for testing the source patient after occupational exposures to blood or body fluids.47,74–78 Rapid tests are more expensive than the EIA, but they offer advantages because they can be performed during initial contact with the patient in the field, clinic, or emergency department; do not require laboratory equipment or specialized technicians; and provide test results during the same visit. Negative test results require no further confirmation and can be reported immediately to the patient. As with any HIV screening test, a reactive rapid test result requires confirmation, but the patient can be counseled about a provisionally positive test result while confirmatory testing is in progress. Performing an EIA after a positive rapid HIV test is optional; supplemental testing with Western blot or IFA is required regardless of the EIA result.79
Three main test formats are used: particle agglutination, immunoconcentration (flow-through), and immunochromatography (lateral flow).80 In particle agglutination tests, latex particles coated with HIV antigen are mixed with patient serum. If HIV antibodies are present, they cross-link the particles and produce agglutination that can be observed visually. Some test kits enhance agglutination by causing the specimen to pass through narrowed capillary channels. Interpretation is simple in strong agglutination reactions, but weak agglutination can be difficult to detect and accurate interpretation is possible only after much experience and practice.81 Automated readers have been developed to improve sensitivity, but with added cost and complexity. Flow-through devices consist of a cartridge with one or more HIV antigens immobilized on a porous membrane. Patient specimen is allowed to flow through the membrane (by gravity or wicked by an absorbent pad on the other side of the porous membrane). Antibody binds to the immobilized antigen, and is detected by the subsequent addition of anti-human IgG bound to a reagent that produces a red or blue color. In the presence of antibody, a dot or visible line forms on the membrane at the antigen location. Many flow-through tests also incorporate anti-human IgG immobilized on the membrane to serve as an internal control: appearance of color at the control location demonstrates that specimen has been added and that the test has been performed correctly. Some tests allow differentiation of HIV-1 from HIV-2 by applying different antigens in different locations on the membrane. The flow-through devices require several steps for application of specimen, wash buffer, and the indicator reagent. Most are suitable for serum or plasma, though some are equipped with a filter or hemolyzing reagent to allow their use with whole blood. Lateral flow devices consist of a nitrocellulose strip, often encased in a plastic cartridge, and incorporate both antigen and signal reagent into the nitrocellulose strip. The specimen (usually followed by a buffer) is added to an absorbent pad. Alternatively, the specimen is diluted in a vial of buffer, to which the device is added. The specimen migrates through the nitrocellulose strip and combines with a colored anti-human IgG signal reagent. The complex then continues to migrate along the strip, over lines where HIV antigen has been applied. A positive reaction results in a colored line on the strip where the HIV antibody–anti-IgG conjugate complex is bound to the HIV antigen. A control line, consisting of immobilized anti-IgG, is often applied to the strip distal to the antigen line. Appearance of a colored line at the control location ensures adequate flow of sample and integrity of the reagents. Most lateral flow devices require only a single step and no additional equipment or refrigeration; test results can be obtained in less than 30 min. Many can be used with multiple types of specimen: finger stick or venipuncture whole blood, plasma, serum, or oral fluid.
Six rapid assays were approved by the US FDA as of July 2006, and many more are available internationally. A listing of commercially available rapid HIV tests is available at http://www.rapid-diagnostics.org/rti-hiv-com.htm. Systematic evaluations have established that most rapid tests adequately detect all subtypes of group M, but performance is more variable with group O and HIV-2.82–92 Limited data from seroconversion panels indicate that analytic sensitivity for early infection is comparable to that of first and second generation EIAs currently licensed by the FDA.84,88 The WHO maintains results of its periodic rapid HIV test evaluations at http://www.who.int/diagnostics_laboratory/publications/evaluations/en/index.html. Sequential algorithms that use combinations of rapid tests for screening and confirmation are recommended by the WHO (Fig. 1-7) and have been used effectively in international settings for years.47,71,93–95 Similar evaluations are underway in the US, and a same-visit confirmation procedure would be a major advance.
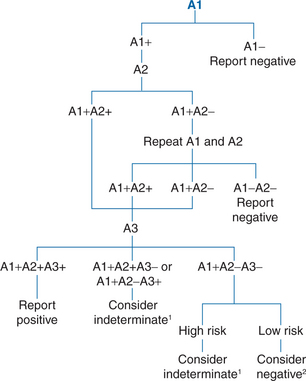
Figure 1-7 Schematic representation of the UNAIDS WHO diagnostic testing algorithm using combinations of screening or rapid tests. A1, A2, and A3 represent 3 different assays.1 Testing should be repeated on a second sample taken after 14 days.2 In the absence of any risk for HIV infection.
Redrawn with permission from World Health Organization, Weekly Epidemiologic Record, vol 72, no 12, 1997, p 84.
Alternative Specimens: Oral Fluid and Urine
For settings where blood collection is impractical, for the needle-phobic, and for improved compliance, tests have been devised to detect HIV antibody in oral fluid and urine. Initially, these tests were not as sensitive or specific as blood tests, but the current tests perform comparably.35,86,87,95–99 Many patients find HIV testing with oral fluid and urine to be more acceptable than conventional blood tests.100,101
Because antibody levels in whole saliva are low, oral fluid HIV tests are designed for use with oral mucosal transudate, secreted at the crevice between the tooth and the gum. This oral fluid contains a quantity of HIV antibody, predominately of the IgG isotype, that is sufficient for testing. When used with oral fluid obtained from both adults and children, commercial HIV assays show sensitivity and specificity comparable to those of tests performed with serum.97,102 Two oral fluid tests have been approved for HIV diagnosis by the FDA. The Orasure test (OraSure Technologies, Bethlehem, PA, USA) uses an oral fluid collection device to obtain antibody-rich oral fluid. After collection, the device is inserted into a vial containing an antibacterial preservative and sent to a laboratory for testing with an EIA and Western blot specifically optimized for use with oral fluid. Compared to serum-based testing, the sensitivity and specificity of the Orasure test process approach 100%.97,98 The Oraquick Advance HIV-½ rapid antibody test, from the same manufacturer, is a lateral flow rapid test that is used to both collect and test oral fluid. Test results can be obtained in less than 30 min, but sensitivity and specificity, though greater than 99%, are slightly lower than those obtained when the same device is used with blood or plasma.99 Relative performance in early seroconversion has not been extensively evaluated.
Several studies have shown that, despite very low IgG levels in urine compared with serum, EIAs can be performed on urine with results that are similar to those with serum.103–106 Both a urine EIA and Western blot (Calypte Biomedical, Inc., Lake Oswego, OR) are FDA approved for HIV-1 diagnosis. However, because sensitivity and specificity with urine are slightly lower than with parallel serum testing,107 reactive HIV test results from urine may require confirmation with serum testing.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree