Esophageal Cancer
Less than 15% of patients diagnosed with esophageal cancer are cured, with approximately half of patients presenting with unresectable or metastatic disease. This chapter reviews the natural history and treatment of esophageal cancer, including anatomy, risk factors, patterns of spread and failure, staging, results of current therapeutic approaches, radiation planning techniques, toxicity data, and future treatment strategies.
 ANATOMY
ANATOMY
The esophagus is a thin-walled, hollow tube approximately 25 cm in length. It is lined with stratified keratinized squamous epithelium, extending from the cricopharyngeus muscle at the level of the cricoid cartilage superiorly to the gastroesophageal junction inferiorly. The lower one-third (5 to 10 cm) of the esophagus may contain glandular elements. Replacement of the stratified squamous epithelium with columnar epithelium is referred to as Barrett’s esophagus, often occurring in the lower one-third. The Z-line refers to the endoscopically visible junction of the squamous and glandular epithelium. The esophageal wall is composed of three layers: the mucosa, submucosa, and muscularis propria (Fig. 53.1). The mucosal layer contains the epithelium, lamina propria, and muscularis mucosae. The epithelium is separated from the lamina propria by a basement membrane. In the portion of esophagus containing columnar-type epithelium, the muscularis mucosae may consist of two layers. The mucosa may be divided into distinct layers, including M1 (epithelium), M2 (lamina propria), and M3 (muscularis mucosae). Similarly, the submucosal layer may be divided into inner (SM1), middle (SM2), and outer (SM3) layers. The muscularis propria consists of a circular inner layer and longitudinal outer layer. The adventitia (periesophageal connective tissue) lies directly on the muscularis propria.1 No serosa is present, facilitating extraesophageal spread of disease.
Although somewhat arbitrary, the esophagus is frequently divided into cervical and thoracic components. The most recent American Joint Committee on Cancer (AJCC) report divides the esophagus into four regions: cervical, upper thoracic, midthoracic, and lower thoracic (Fig. 53.2).1 The cervical esophagus begins at the cricopharyngeus muscle (approximately the C7 level or 15 cm from the incisors) and extends to the thoracic inlet (at approximately the T3 level or at approximately 20 cm from the incisors, at the level of the suprasternal notch), and therefore lies within the neck. The thoracic esophagus extends from approximately the level of T3 (beginning at about 20 cm) to T10 or T11.1,2 The upper thoracic esophagus is bordered superiorly by the thoracic inlet and inferiorly by the lower border of the azygos vein, extending from approximately 20 to 25 cm. Radiographically, tumors in this location would be located between the sternal notch and azygos vein. The middle thoracic esophagus extends from the lower border of the azygos vein to the inferior pulmonary veins, extending from approximately 25 to 30 cm. The lower thoracic esophagus extends from the inferior pulmonary veins and to the stomach and is inclusive of the gastroesophageal junction, typically extending from approximately 30 to 40 cm. Endoscopically, the gastroesophageal (GE) junction is often defined as the point where the first gastric fold is encountered, although this may be a “theoretical” landmark. The location of the GE junction can be accurately defined histologically as the squamocolumnar junction. In the most recent AJCC staging system, cancers with an epicenter in the lower thoracic esophagus, gastroesophageal junction, or within the proximal 5 cm of the stomach (i.e., cardia) and extending up to the GE junction or esophagus are staged as an adenocarcinoma of the esophagus. If the epicenter is >5 cm distal to the gastroesophageal junction or within 5 cm of the gastroesophageal junction but does not extend to the junction/esophagus, tumors are classified as stomach cancers. Useful landmarks in reference to endoscopy include the carina (∼25 cm from the incisors) and gastroesophageal junction (∼40 cm from the incisors).
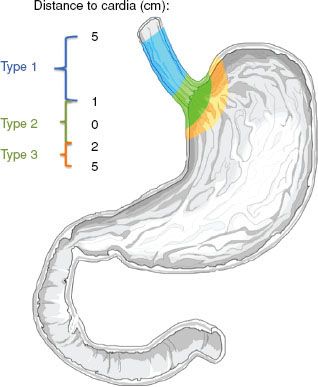
Siewert et al.3,4 characterized cancer involving the gastroesophageal junction according to the location of the tumor (Fig. 53.3). If the tumor center is located from >1 cm up to 5 cm above the gastroesophageal junction (Z-line), the tumor is classified as a type I adenocarcinoma of the distal esophagus. If the tumor center is located within 1 cm cephalad to 2 cm caudad to the gastroesophageal junction, it is classified as type II. If the tumor center is located >2 cm below the gastroesophageal junction, the tumor is classified as type III. However, locally advanced/bulky tumors can make it difficult to accurately distinguish where tumors originated in relationship to the GE junction.
FIGURE 53.1. Diagram of esophageal wall. (Used with the permission of the American Joint Committee on Cancer, Chicago, Illinois. The original source for this material is American Joint Committee on Cancer. AJCC cancer staging handbook, 7th ed. New York: Springer, 2010, published by Springer Science and Business Media LLC, www.springerlink.com.)
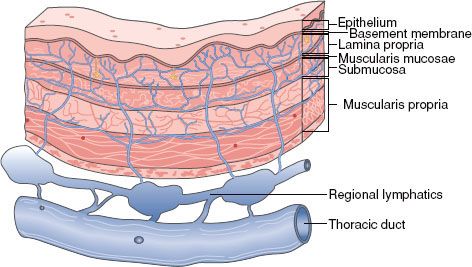
FIGURE 53.2. Anatomy of the esophagus. Note the lengths of various segments of the esophagus as measured from the upper central incisors. (Used with the permission of the American Joint Committee on Cancer, Chicago, Illinois. The original source for this material is American Joint Committee on Cancer. AJCC cancer staging handbook, 7th ed. New York: Springer, 2010, published by Springer Science and Business Media LLC, www.springerlink.com.)

FIGURE 53.3. Siewert classification of the gastroesophageal junction cancers according to the location of the tumor. (From Matzinger O, Gerber E, Bernstein Z, et al. EORTC-ROG expert opinion: radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother Oncol 2009;92:164–175; copyright 2009, with permission from Elsevier.)
Lymphatic Drainage
The esophagus has an extensive, longitudinal interconnecting system of lymphatics. The esophageal lymphatic network is primarily located within the submucosa; however, channels are also present within the lamina propria, facilitating spread of even superficial cancers of the esophagus involving the mucosa. In addition to these longitudinal lymphatics, intramural lymphatics may traverse the muscularis propria, facilitating tumor spread to regional lymphatic channels and paraesophageal nodes. Supporting this, autopsy series have demonstrated a relatively high incidence of directly draining channels extending from the submucosa lymphatics into the thoracic duct (Fig. 53.1), facilitating systemic spread. Lymph can travel the entire length of the esophagus before draining into lymph nodes,2 and thus the entire esophagus is at potential risk for lymphatic involvement. Up to 8 cm or more of ‘normal’ tissue can exist between gross tumor and micrometastases “skip areas” secondary to this extensive lymphatic network.5 In addition, as many as 71% of frozen tissue sections scored as margin negative by conventional histopathology show involvement by lymphatic micrometastases with immunohistochemistry.6 Lymphatics of the esophagus drain into nodes that usually follow arteries, including the inferior thyroid artery, the bronchial and esophageal arteries, and the left gastric artery (celiac axis).7
Epidemiology and Risk Factors
Esophageal carcinoma accounts for approximately 6% of all gastrointestinal malignancies. In 2012, there will be an estimated 17,460 new patients diagnosed with esophageal cancer in the United States and 15,070 deaths. Most cases occur in males, at a rate of 4:1 relative to females.8,9
The incidence of esophageal carcinoma varies according to geography. The highest incidence occurs in Linxian, China, Russia, and the Caspian region of Iran. The incidence there is 100/100,000 persons. In addition, there are many differences within regions within those countries. In areas such as northern France, Kazakhstan, and South Africa incidence can be as high as 50 to 99/100,000. Although the reasons for the geographic discrepancy are unknown, some reports have linked the arid climate and alkaline soil with these high-risk areas, as well as the ingestion of nitrosamines, and inversely to the consumption of riboflavin, nicotinic acid, magnesium, and zinc.10,11 In the United States, the incidence rate among males is <5/100,000. Over the last 20 years, there has been an increase in the incidence of adenocarcinoma at a rate of 5% to 10% per year. This is a more rapid increase than that for any other cancer.12 In 1987, adenocarcinoma was reported to represent 34% and 12% of esophageal cancers in White men and women versus 3% and 1% for African American men and women, respectively.12 As of 1998, esophageal adenocarcinoma accounted for almost 55% of all diagnosed cases in White men. African American men are more frequently diagnosed with squamous cell carcinoma.12–14
In North America and Western Europe, alcohol and tobacco use are the major risk factors for squamous cell carcinoma, accounting for 80% to 90% of cases.15 Reports have described the relative risk of esophageal cancer by the amount of alcohol and tobacco consumed, including a relative risk of 155:1 when consuming >30 g/day of tobacco along with 121 g/day of alcohol.16
Diets of scant amounts of fruits, vegetables, and animal products are associated with increases in squamous cell carcinoma.17 Patients with Plummer-Vinson (Paterson-Kelly) syndrome, a condition characterized by iron-deficiency anemia and low riboflavin levels, are at an increased risk for oral cavity, hypopharyngeal, and esophageal cancer. In addition, dietary intake of nitrosamines, nitrosamides, and N-nitroso compounds has been implicated in esophageal carcinoma. Examples of nitrate-rich foods include pickled vegetables, alcoholic beverages, cured meats, and fish.18,19
Other risk factors associated with esophageal carcinoma include achalasia and tylosis. Achalasia of long duration (25 years) is associated with a 5% incidence of squamous cell carcinoma.20,21 Patients with tylosis (hyperkeratosis of the palms and soles and papilloma of the esophagus) have a reported 38% risk in developing esophageal cancer at a mean age of 45 years.22 In addition, carcinoma of the esophagus occurs in 2% to 4% of patients with head and neck cancer.
Risk factors leading to the development of adenocarcinoma of the esophagus are being increasingly understood. Most esophageal adenocarcinomas tend to arise from the metaplastic columnar-lined epithelium known as Barrett’s esophagus.23 Severe and long-standing gastroesophageal reflux disease has clearly been shown to be a significant risk factor for Barrett’s esophagus, which may lead to adenocarcinoma. It has been estimated that patients with long-standing severe reflux have a 44-fold risk of developing adenocarcinoma.24 Tobacco use is a more moderate risk factor for adenocarcinoma development. Smokers appear to have a twofold to threefold greater risk for developing esophageal adenocarcinoma than nonsmokers.25,26 The relative risk of esophageal adenocarcinoma persists to three decades following smoking cessation, in contrast to a significant decline in similar patients with squamous cell carcinoma.27 Obesity has also been linked to a threefold to fourfold risk of adenocarcinoma, possibly due to an increased risk of reflux.28 It has been estimated that a middle-aged patient with Barrett’s esophagus has a 10% to 15% risk of developing esophageal adenocarcinoma during his or her lifetime.29
Although many risk factors are associated with esophageal carcinoma, few studies have demonstrated a causal relationship leading to pathogenesis. Motesano et al.30 reported possible genetic abnormalities involved in the genesis of esophageal cancer. In addition, possible differences in mechanisms of pathogenesis for squamous cell carcinoma and adenocarcinoma were described. Genetic abnormalities in squamous cell carcinoma include p53 mutations and multiple allelic losses at 3p and 9q, with amplification of cyclin D1 and epidermal growth factor receptor (EGFR). These mutations lead to cell hyperplasia, low- and high-grade dysplasia, and, ultimately, squamous cell carcinoma. In contrast, genetic abnormalities in adenocarcinoma include overexpression of p53, multiple allelic losses at 17p, 5q, and 13q, and amplification and overexpression of EGFR and human epidermal growth factor receptor 2 (HER-2). These abnormalities may be involved in the stepwise development of Barrett’s esophagus, dysplasia, and, ultimately, adenocarcinoma. These differences suggest that squamous cell carcinoma and adenocarcinoma have different series of genetic mutations as etiologies, but these abnormalities occur in 23% to 94% of tumors studied.
TABLE 53.1 DISTRIBUTION OF METASTASES BY ANATOMIC SITE
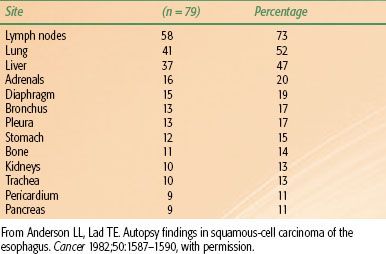
FIGURE 53.4. Positive lymph node distribution according to the location of the primary tumor, squamous cell carcinoma. (From Huang W, Li B, Gong H, et al. Pattern of lymph node metastases and its implication in radiotherapeutic clinical target volume in patients with thoracic esophageal squamous cell carcinoma: a report of 1077 cases. Radiother Oncol 2010;95:229–233; with permission from Elsevier.)

 NATURAL HISTORY AND PATTERNS OF SPREAD
NATURAL HISTORY AND PATTERNS OF SPREAD
Squamous cell carcinoma is characterized by extensive local growth and proclivity to lymph node metastases. Because the esophagus has no covering serosa, direct invasion of contiguous structures may occur early. Lesions in the upper esophagus can impinge on or invade the recurrent laryngeal nerves, carotid arteries, and trachea. If extraesophageal extension occurs in the mediastinum, tracheoesophageal or bronchoesophageal fistula may occur. Tumors in the lower one-third of the esophagus can invade the aorta or pericardium, resulting in mediastinitis, massive hemorrhage, or empyema.
A review correlating the incidence of lymph node metastases with depth of penetration revealed that 18% of patients with spread to the submucosa had lymph node involvement.31 For T1 lesions, the reported incidence of nodal spread is 14% to 21%; for T2 lesions, this rises to 38% to 60%.32,33 The location of involved lymph nodes is influenced by the origin of the primary tumor. At autopsy, lymph node metastases are found in approximately 70% of patients.34,35–36 In patients with cervical lesions, lymph node metastases to the abdominal lymph nodes are rare. Distant metastasis can occur at almost any site (Table 53.1).37
A review of 1,077 patients with squamous cell carcinoma of the thoracic esophagus undergoing esophagectomy further characterized patterns of nodal spread. Primary disease was located in the upper (5%), middle (63%), and lower (32%) thoracic esophagus. In total, 47% of patients had lymph node metastases. On multivariate analysis, T stage, tumoral length, and degree of differentiation significantly correlated with incidence of lymph node metastases. In approximately 6% of cases, skip metastasis (distant lymph node metastases without regional lymph node metastasis) occurred, usually in patients with poorly differentiated, large and deeply invasive tumors. Of involved nodes, 37% were macroscopically involved versus 63% microscopically involved, indicating that most lymph node metastases would be below the resolution of detection using contemporary imaging tools. Lymph node involvement was grouped into five categories; cervical, thoracic upper mediastinum, thoracic middle mediastinum, thoracic lower mediastinum, and abdominal lymph nodes. Figure 53.4 shows the incidence of nodal metastases based on primary tumor location.38
For lower esophageal and gastroesophageal junctional adenocarcinomas, approximately 70% of patients will have nodal metastases at presentation. This is influenced by tumoral depth of penetration, with nearly all T3 and T4 lesions exhibiting metastases in surgical series. Pathologic resection data demonstrated rates of lymphatic involvement for lower esophageal and GE junctional tumors of 45%, 85%, and 100% for T2, T3, and T4 tumors, respectively (Fig. 53.5).33 In patients with lower esophageal cancer, involvement of both mediastinal and abdominal lymph nodes is common (Fig. 53.6).33 The primary direction for lymphatic flow for the lower esophagus is toward the abdomen. According to the classification by Siewert, nodal metastases are often seen in the mediastinum and abdomen for type I tumors, whereas type III tumors metastasize almost exclusively inferiorly, toward the celiac axis. Type II tumors are intermediate, preferentially spreading inferiorly and less frequently into the mediastinum. The primary value in the Siewert classification is to the guidance of appropriate type surgery (i.e., type I tumors are generally treated with esophagectomy and mediastinal lymph node resection, with types II and III approached through the abdomen), although it may be useful in radiation field design as well.3 Generally speaking, the incidence of abdominal nodal involvement increases as one proceeds distally in the esophagus to the gastroesophageal junction. For patients with tumors arising from the gastroesophageal junction, mediastinal involvement is less common. Nodal metastases above the level of the carina are rare in lower esophageal and junctional tumors.39 In addition, histologic analyses of lower esophagus and gastroesophageal junction adenocarcinoma specimens suggest that many patients without nodal involvement on conventional histopathology actually have involvement when assessed by immunohistochemistry.40
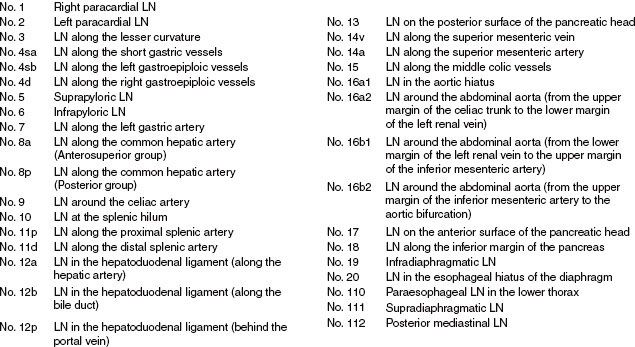
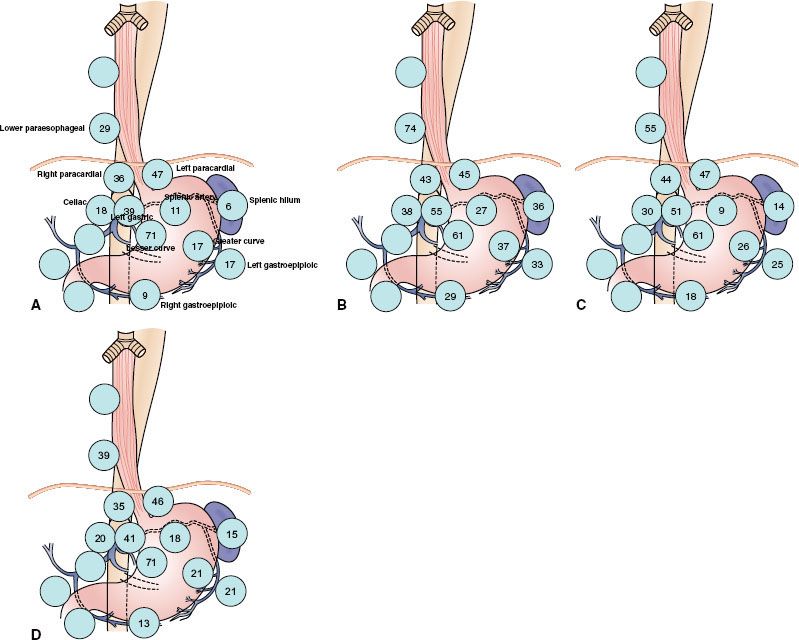
Extensive nodal mapping has been performed by Japanese investigators, who have devised the Japanese Gastric Cancer Association Classification (Fig. 53.7).41 Using this system of nodal classification, investigators from Erlangen evaluated 326 patients with esophagogastric junction carcinoma undergoing primary resection. Tumors were stratified as AEG type I (distal esophagus), II (gastric cardia), and III (subcardia). Note there was significant overlap in the majority of tumors for all stages, with an overall incidence of lymph node metastasis of 71%. In T1 patients, only 17% exhibited lymph node metastasis, whereas 78%, 86%, and 90% of T2, T3, and T4, respectively, had involved nodes. Figure 53.8 shows varying patterns of nodal spread based on T stage as well as AEG location. Additional generalizations from this study include the following: (a) lymph vascular invasion was highly predictive of nodal spread and (b) proximal extension of type II and III tumors into the distal esophagus (particularly beyond the Z-line) predicted an increasing incidence of paraesophageal lymph node involvement.42
FIGURE 53.5. The incidence of nodal metastases related to depth of tumor invasion, adenocarcinoma. (From Dresner SM, Lamb PJ, Bennett MK, et al. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery 2001;129:103–109; with permission form Elsevier.)

FIGURE 53.6. Distribution of nodal metastases by frequency of site involved for adenocarcinoma. (From Dresner SM, Lamb PJ, Bennett MK, et al. The pattern of metastatic lymph node dissemination from adenocarcinoma of the esophagogastric junction. Surgery 2001;129:103–109; with permission form Elsevier.)

 LOCAL FAILURE
LOCAL FAILURE
Aisner et al.43 and LePrise et al.44 reviewed the patterns of failure in esophageal cancer after radical irradiation, radical surgery, or a combination of both (Table 53.2). These data suggest that high rates of local recurrence occur when either radiation therapy or surgery alone is used. In a series at the University of Pennsylvania and Fox Chase Cancer Center of patients with adenocarcinoma of the esophagus and gastroesophageal junction treated with surgery alone, the local-regional recurrence rate was 77%.45 In contemporary randomized trials, local failure rates with surgery alone range from 32% to 45%.46–49 Similarly, data from recent randomized trials of esophageal cancer using “definitive” chemoradiation also indicates that local failure is a major cause of failure, with approximately 50% of patients failing locally (Table 53.3). In many trials, patterns of failure are reported as first site of recurrence. This fact, along with infrequent posttherapy imaging and inability to detect subclinical recurrences, likely leads to underestimates of local recurrence rates. These data clearly emphasize the need for improvements in local treatment modalities.
 CLINICAL PRESENTATION
CLINICAL PRESENTATION
Symptoms of esophageal cancer often start 3 to 4 months before diagnosis. Location of the primary tumor in the esophagus may influence presenting symptoms. Dysphagia is seen in >90% of patients regardless of location. Odynophagia is present in up to 50% of patients.50 Weight loss is common, with 40% to 70% of patients reporting a loss of >5% of total body weight. This extent of weight loss has been associated with a worse prognosis. Less frequent symptoms may include vague chest pain, hoarseness, cough, and glossopharyngeal neuralgia.51
Advanced lesions can produce signs and symptoms from tumor invasion into local structures. Hematemesis, hemoptysis, melena, dyspnea, and persistent cough secondary to tracheoesophageal or bronchoesophageal fistula may occur. Compression or invasion of the left recurrent laryngeal nerve or the phrenic nerves can cause dysphonia or hemidiaphragm paralysis. Superior vena cava syndrome and Horner’s syndrome can also occur for very advanced lesions. Pleural effusion and exsanguination resulting from aortic communication may also be seen.50 Abdominal and back pain may occur with celiac axis nodal involvement with lower esophageal tumors.
FIGURE 53.7. Locations of lymph node stations. (From Matzinger O, Gerber E, Bernstein Z, et al. EORTC-ROG expert opinion: radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother Oncol 2009;92:164–175; with permission from Elsevier.)
 DIAGNOSTIC WORKUP
DIAGNOSTIC WORKUP
After a thorough history and physical examination, all patients with suspected esophageal cancer should have a workup similar to that outlined in Table 53.4. Attention should be paid to cervical and supraclavicular lymph nodes. Basic blood counts and a metabolic panel with liver function tests should be obtained.
TABLE 53.2 PATTERNS OF FAILURE IN ESOPHAGEAL CANCER
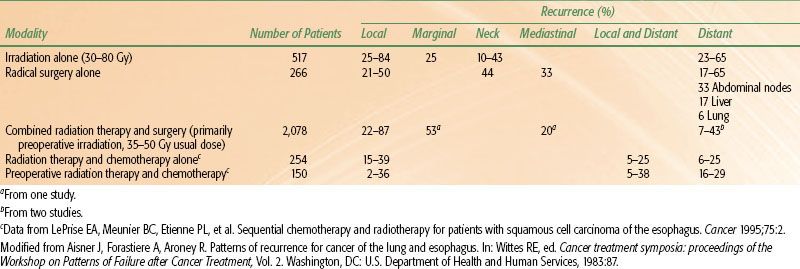
FIGURE 53.8. A: Frequency of nodal involvement for T2 tumors. B: Frequency of nodal involvement for T3-4 tumors. C: Frequency of involvement for AEG type I tumors. D: Frequency of nodal involvement for AEG type II/III tumors. (Based on data from Meier et al.42)
Although the esophagogram may be used to define lesion extent, endoscopy is the best tool to diagnose and define the extent of the lesion. During flexible endoscopy, biopsies and brushings should be taken of the primary site and suspicious areas harboring satellites or submucosal spread. In addition, accurate endoscopic measurement and characterization of tumor and gastroesophageal junction in relation to the incisors facilitates radiation treatment planning. Examination with panendoscopy of the oral cavity, pharynx, larynx, and tracheobronchial tree may also be performed at the time of esophagoscopy in patients with squamous cell carcinomas, given the high incidence of second tumors in the head and neck and upper airway.2 In addition, bronchoscopy should generally be performed in patients with proximal malignancies to evaluate for the presence of tracheal or carinal invasion, particularly for patients with tumors abutting these structures on computed tomography (CT). CT of the thorax and abdomen is critical to identify metastases to the liver, upper abdominal nodes, or adrenals. However, CT may not adequately assess periesophageal lymph node involvement or accurately define the true extent of the primary tumor.52,53 Conventional CT scan can accurately determine resectability in only 65% to 85% of cases. Furthermore, CT accurately predicts T stage in approximately 70% of cases and nodal involvement in only 50% to 70% of cases.54–56
To more accurately assess periesophageal and celiac lymph node involvement and transmural extent of disease, endoscopic ultrasonography (EUS) should be performed. EUS provides accuracy rates of 85% to 90% for tumor invasion (T stage) and 75% to 80% for lymph node metastases when matched to surgical pathology.57–60 However, the accuracy of endoscopic ultrasound following neoadjuvant therapy is significantly less, ranging from 27% to 48% for T staging and 38% to 71% for N staging. This is possibly due to the failure to discriminate tumor from postradiation inflammation and fibrosis.61–63
Surgical staging procedures, including thoracoscopy, mediastinoscopy, and laparoscopy, may provide additional staging information and are considered in selected patients at some institutions.64 Laparoscopy would primarily be used for tumors at the gastroesophageal junction.
Patients with significant obstruction with inability to maintain their weight may require placement of a feeding tube. If surgery is planned, gastric tube placement is generally avoided, given that the stomach will ultimately serve as the “neoesophagus” following resection.
More recently, positron emission tomography (PET) has proven to be a valuable staging tool in esophageal cancer patients. The addition of PET to standard staging studies such as CT can improve the accuracy of detecting stage III and stage IV disease by 23% and 18%, respectively.65,66 Overall, it is estimated that PET will detect distant metastatic disease in approximately 20% of patients who are considered to have local regional disease only by CT. However, PET also appears to have a lower accuracy in detecting local nodal disease compared to CT alone or in combination with endoscopic ultrasound. Of importance, emerging data suggest that PET can be used to predict response to therapy, with “PET responders” experiencing significantly improved outcomes compared to “nonresponders.”67 In addition, PET has been used to predict response to treatment early during the treatment course. This has led to ongoing investigation of early treatment response as measured by PET as a surrogate for therapeutic efficacy and clinical outcomes.68
TABLE 53.3 LOCAL FAILURE RATES FROM RANDOMIZED TRIALS EVALUATING CHEMORADIATION ALONE IN ESOPHAGEAL CANCER
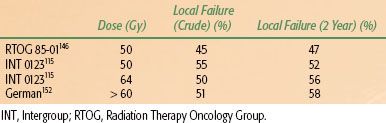
TABLE 53.4 DIAGNOSTIC WORKUP FOR ESOPHAGEAL CANCER

 STAGING SYSTEMS
STAGING SYSTEMS
Esophageal staging can be based on pathologic or clinical criteria. Pathologic staging is performed after invasive procedures, including esophagectomy, mediastinotomy, or thoracotomy. Clinical staging is often employed with “definitive” and neoadjuvant chemoradiation approaches and is less accurate. With the combination of CT, PET, and EUS, clinical staging closely correlates with pathologic stage. Note that the most recent AJCC staging system takes into consideration tumor type and grade, as well as involved number of lymph nodes (Table 53.5).
 PATHOLOGIC CLASSIFICATION
PATHOLOGIC CLASSIFICATION
Squamous cell carcinoma and adenocarcinoma comprise 95% of all esophageal tumors, although other rare histologic subtypes are occasionally seen (Table 53.6).69
Given that there has been a rise in adenocarcinoma compared to squamous cell carcinoma, examinations of outcomes by histology have been performed. Some series have reported that patients with squamous cell carcinomas have improved survival,70 whereas others have suggested that patients with adenocarcinoma have an improved survival71 and others report no survival differences. Overall there can be no direct comparison until randomized studies are done with stratification based on histology.
Pseudosarcoma is a variant of a poorly differentiated squamous cell carcinoma with spindle-shaped cells in the stroma resembling fibroblasts. Verrucous carcinoma is a well-differentiated, papillary variant of squamous cell carcinoma.2 Squamous cell carcinoma in situ is rarely seen in the United States and should be distinguished from dysplasia.72–74
Adenocarcinoma may arise from foci of ectopic gastric mucosa or intrinsic esophageal glands. However, as described previously, it is believed the vast majority arise from Barrett’s esophagus. If a focus of squamous cell metaplasia is found in an adenocarcinoma, the tumor may be referred to as an adenoacanthoma.75
Adenoid cystic carcinomas are rare, with an incidence of 0.75%. Patients with this malignancy present around the sixth decade of life and have a poor median survival.76 Mucoepidermoid tumors (adenosquamous carcinomas) are more aggressive and carry a poor prognosis.77 The incidence of small-cell carcinoma is approximately 2%. Patients with these malignancies often present in the sixth to eighth decades of life, and the lesion is usually located in the middle to lower esophagus in males.78,79 These are believed to originate in the argyrophilic cells in the esophagus and may produce paraneoplastic syndromes, such as antidiuretic hormone secretion and hypercalcemia.80 The clinical course of small-cell carcinoma is similar to that of small-cell carcinoma of the lung and may be responsive to chemotherapy and radiation therapy.77,79
Nonepithelial tumors of the esophagus are rare. Among these, leiomyosarcomas are the most common. Twenty-five percent of patients with this tumor present with metastases.81–83 Histologically, these tumors have interlacing bundles of spindle-shaped cells. Less aggressive forms have fewer mitotic figures and less anaplasia. Prognosis has been reported to be more favorable than that of squamous cell carcinoma.77 In patients with Kaposi’s sarcoma, gastrointestinal involvement of the esophagus can be seen.84
Malignant melanoma is rare and can occur as a primary esophageal tumor or as a metastasis. These lesions are usually large and often covered by intact squamous mucosa with focal areas of ulceration. Spread is usually submucosal. Mean survival is approximately 7 months.85,86 Lymphoma comprises approximately 1% of esophageal malignancies. It is usually associated with direct extension from other organs, although primary esophageal lymphoma has been reported.87
TABLE 53.5 AMERICAN JOINT COMMITTEE ON CANCER 2010 ESOPHAGEAL CANCER STAGING SYSTEM
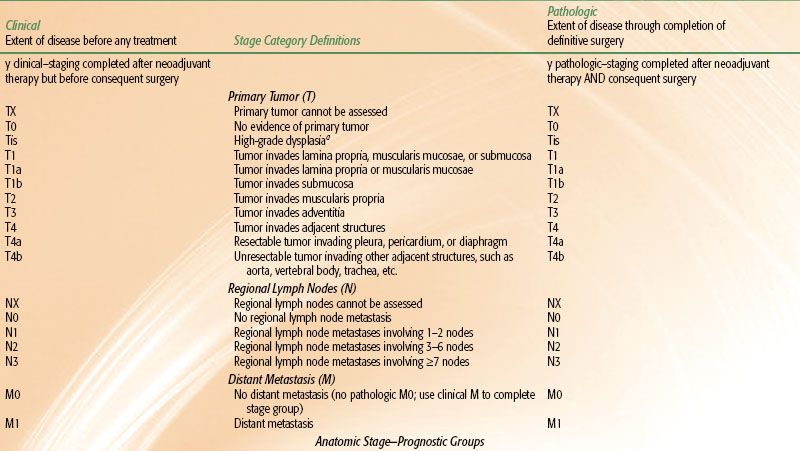
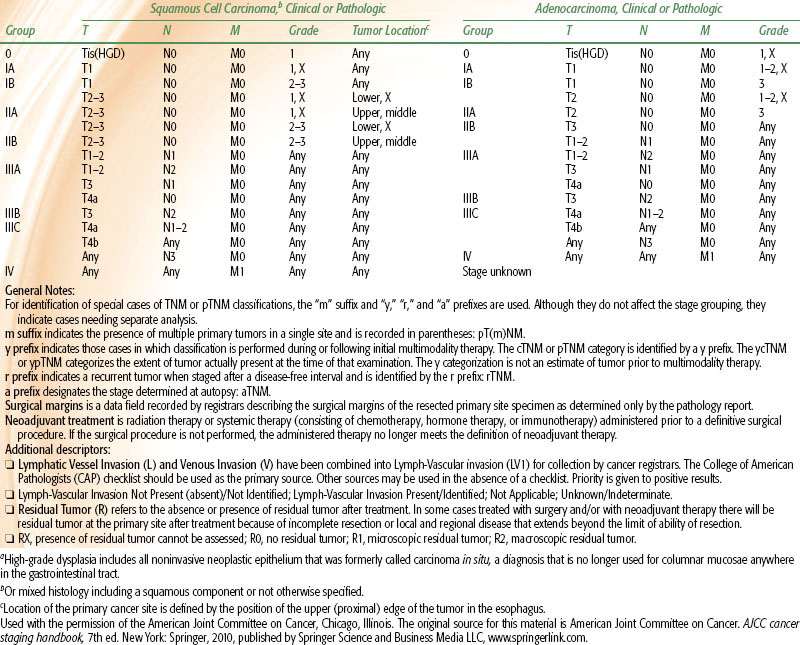
TABLE 53.6 PATHOLOGIC CLASSIFICATION OF MALIGNANT ESOPHAGEAL TUMORS

 PROGNOSTIC FACTORS
PROGNOSTIC FACTORS
According to the Union Internationale Contre le Cancer/American Joint Cancer Committee seventh-edition staging system, R-status, age, and histologic subtype were independent prognostic factors of survival, whereas tumor grade and site were not.88 It should be recalled that these data consider patients treated with surgery alone and as such do not apply to patients receiving chemotherapy or radiotherapy. A study published by Nomura et al.89 evaluated 301 patients treated with chemoradiotherapy showed that T stage, M stage, and gender were prognostic factors in multivariate analysis.
In addition to stage, other factors portend outcome. Tumor length/size may also impact outcome. In a study of 582 patients undergoing surgical resection as primary treatment, tumoral length adversely affected survival, with 5-year survival rates of 77%, 48%, 38%, and 23% for tumor lengths of 1, 2, 3, or >3 cm, respectively (p < .001).90 Of note, length was not prognostic if patients were N+ or M+. Similarly, in adenocarcinoma patients, 5-year survival rates have been shown to be significantly higher for patients with tumors ≤2 cm compared to those >2 cm.91
In another large study from the Mayo Clinic, clinicopathologic factors that affected prognosis included T and N status, tumor grade, age >76 years, extracapsular lymph node extension, and the absence of chemotherapy or radiotherapy; anatomic location did not influence survival.92 In addition, weight loss and low overall performance status also indicate poor prognosis.93 Deep ulceration of the tumor, sinus tract formation, and fistula formation are other poor prognostic factors.50
Obtaining uninvolved pathologic margins at resection is of significant importance with regard to long-term outcome. An Intergroup study (discussed later) evaluating chemotherapy preceding and following esophagectomy showed that outcomes were similar in patients undergoing R1 resection (positive microscopic margins) or R2 resection (gross residual disease) or patients not undergoing resection at all. Only patients undergoing R0 resection (uninvolved margins) had a substantial chance of long-term disease-free survival.47 A patterns-of-care survey examined the outcomes of patients with adenocarcinoma and squamous cell carcinoma of the esophagus between 1996 and 1999. Patients were treated with radiation therapy across 59 institutions. On multivariate analysis, significant improvements in survival were seen in patients treated at centers with ≥500 new cancer patients per year compared to centers seeing <500 (hazard ratio 1.32; p = 0.03).94
 GENERAL MANAGEMENT
GENERAL MANAGEMENT
Treatment for esophageal carcinoma is characterized as curative or palliative. According to one historical study,95 only 20% patients present with cancer of the esophagus that is truly localized to the esophagus, indicating that at the time of diagnosis, approximately 80% patients have either locally advanced or distant disease.
Surgery with Curative Intent
Surgery of the thoracic esophagus usually requires a subtotal or total esophagectomy and is usually undertaken for lesions of the middle to lower one-third of the thoracic esophagus and gastroesophageal junction. Patients with stage I to III are often considered for potentially curative resection; however, aortic, tracheal, heart, or great vessel invasion may preclude resection. Esophagectomy may be accomplished by a number of techniques, including a transhiatal esophagectomy, right thoracotomy with laparotomy with intrathoracic anastomosis (Ivor-Lewis esophagogastrectomy), right thoracotomy with laparotomy with cervical anastomosis (McKeown esophagogastrectomy), left thoracotomy, or radical esophagectomy via open or laparoscopic approaches. Each technique has its advantages and disadvantages. In general, advantages of the transthoracic approach include better visualization with access and resection of the upper two-thirds of the esophagus and mediastinal lymph nodes. Alternatively, the transhiatal approach has less morbidity than thoracotomy (including respiratory compromise) with easier access to anastomotic leaks (neck vs. thorax). In any instance, achievement of negative margins at resection has been reported to be a significant prognostic factor and should be the goal of esophageal resection. Exemplifying this, in a study of 500 patients undergoing transthoracic resection, patients undergoing margin-negative resection had a 5-year survival rate of 29% versus no 5-year survivors in patients with involved margins.96
The Ivor-Lewis procedure is the classic approach to expose mid-esophageal lesions. A left thoracotomy procedure exposes lesions of the gastroesophageal junction. Transhiatal esophagectomy is performed without a thoracotomy and is useful in lower esophageal lesions, although direct visualization and dissection of varying mediastinal lymph nodes cannot be achieved. The optimal surgical approach is unknown. A randomized trial comparing transhiatal versus transthoracic approaches in patients with adenocarcinoma showed no significant survival advantage to the latter, although a possible trend was noted (5-year survival, 29% vs. 39%). However, perioperative mortality was also increased with the transthoracic approach.46
Laparotomy can be performed before or concurrently with esophagectomy to rule out any disease below the diaphragm. Multiple reconstruction options are available following definitive surgery; esophagogastrostomy is the most widely used, using the stomach as a conduit to replace the esophagus. Patients with significant obstruction and inability to maintain their weight often require placement of feeding jejunostomy. If possible surgery is planned, gastric tube placement is generally avoided, given that the stomach will ultimately serve as the “neoesophagus” following resection. Colon interposition, preferably with the left colon, can also be used; however, this approach is generally reserved for patients who have previously undergone gastric surgery or other procedures that have devascularized the stomach.
Squamous cell carcinoma of the cervical esophagus presents a difficult management situation. Proximal esophageal tumors <5 cm from the cricopharyngeus are generally treated with definitive chemoradiotherapy. If surgery is performed, resection of portions of the pharynx, the entire larynx, thyroid gland, and the proximal esophagus is often required. Radical neck dissections are also carried out.50 Because of the significant morbidity and loss of organ function with surgery, chemoradiation alone has been frequently employed. The survival probability with definitive chemoradiotherapy is similar, without the major functional impairments, morbidity, and mortality associated with surgery.97
Curative Combination Therapy
In the treatment of patients with esophageal cancer, an approach of radiation therapy with concurrent chemotherapy, with or without surgery, is frequently adopted. Multiagent chemotherapy with cisplatin and 5-fluorouracil (5-FU) is used most frequently, although combinations of other agents (e.g., paclitaxel and carboplatin) are being increasingly used. Additional taxanes, topoisomerase inhibitors, and anti-epidermal growth factor receptor inhibitors with radiation therapy are under investigation.
Palliative Treatment
Palliative treatment is frequently used for the relief of symptoms of esophageal carcinoma, especially dysphagia.98 Surgical palliation involves resection and reconstruction, if possible, removing the bulk of the disease, potentially preventing abscess and fistula formation, as well as bleeding. Substernal bypass with the colon or entire stomach has also been carried out.98 However, given the poor prognosis in patients with advanced disease and morbidity associated with resection, a surgical approach is not commonly adopted and should be avoided in patients who can be managed with nonsurgical modalities.
Endoscopic dilatation is a reasonable alternative. When the lumen of the esophagus is dilated to 15 mm, dysphagia is often no longer experienced. Repeat dilatation is often required.50 Esophageal stenting with either conventional plastic stents or metallic self-expanding stents can also be used to maintain patency.99
Palliative irradiation is frequently used to control the primary disease, as well as distant metastases. Resolution of symptoms, especially pain and dysphagia, can be accomplished in up to 80% of patients. Palliative treatment regimens range from 30 Gy over 2 weeks51 to 50 Gy over 5 weeks. Laser ablation with or without intraluminal brachytherapy can be used. The addition of 3 fractions of 7 Gy each can improve the stenosis-free interval and prevent obstruction.99
 RADIATION THERAPY TECHNIQUES
RADIATION THERAPY TECHNIQUES
Simulation
When patients are simulated, the radiation oncologist should know the extent of disease based on imaging (barium swallow, CT, PET), as well as on endoscopy. CT simulation is appropriate for treatment planning. During simulation, the patient is positioned, straightened, and immobilized on the simulation table. An immobilization device is used to minimize variation in daily setup. Arms are generally placed overhead and knee support underneath the legs. Palpable neck disease should be marked with a radiopaque wire. The administration of oral contrast to delineate the esophagus is generally used and helps to define the extent of mucosal irregularity. For GE junctional tumors (particularly with significant gastric involvement), it may be advisable to have the patient come in with an empty stomach. For cervical and upper thoracic lesions, an immobilization mask may assist in creating a reproducible position. Some authors have also advocated that mid-esophageal primaries be simulated in prone position to maximize distance between the target volumes and spinal cord. The patient is placed on the CT simulator in the treatment position, and a scan of the entire area of interest with margin is obtained. At minimum, 3- to 5-mm slices should be used, allowing accurate tumor characterization, as well as improved quality of digitally reconstructed radiographs. If patients lose >10% of their body weight during therapy, consideration should be given to repeat CT planning. Arterial phase intravenous contrast is generally used to delineate mediastinal and abdominal vascular nodal basins, including the celiac axis and to allow the radiation oncologist to discern normal vasculature from other adjacent normal structures, and potential adenopathy. The tumor and vital structures are then outlined on each slice on the treatment planning system, enabling a three-dimensional treatment plan to be generated. The use of respiratory gating or breath-hold techniques may help to reduce target motion with respiration and, therefore, avoid normal-tissue irradiation associated with larger margins used in free-breathing approaches, particularly for lower esophageal cancers. Additional techniques to minimize physiologic motion include abdominal compression devices. Four-dimensional CT scan may be appropriate to assess tumoral motion, facilitating appropriate margin placement on the target volumes, particularly in lower esophageal tumors and/or disease involving the stomach.
Treatment Planning
Target Design
In the design of radiation fields for esophageal cancer, it is important to define varying target volumes, including gross disease as well as potential areas of subclinical involvement (i.e., the gross tumor volumes [GTVs] and clinical target volumes [CTVs], respectively). Definition of gross tumor volume is based on multiple studies, including endoscopic descriptions (from both esophagogastroduodenoscopy [EGD] and EUS). The proximal and distal aspects of the tumor should be standardly defined by the gastroenterologist based on distance from the incisors, as well as relationship to varying landmarks as measured from the incisors (e.g., the GE junction). The radiation oncologist is able to use these measurements to help correlate with disease extent visualized on planning CT scan, using varying anatomic landmarks (e.g., the GE junction, which is frequently located ∼40 cm from the incisors in many patients), as well as carina (which is frequently located at ∼25 cm in most patients) to help more accurately define the GTV. Esophageal wall thickening correlating to the gross tumor volume can frequently be visualized on diagnostic and radiation planning CT. Similarly, EUS appears to be the most reliable test in detecting lymphadenopathy related to nodal spread. As in EGD, the endoscopist should be encouraged to accurately define not only the primary disease extent on EUS as measured by distances from the incisors, but also depth of penetration and potential involvement of adjacent structures, which can also be used to help guide GTV delineation. Similarly, EUS may detect lymph nodes that may not be appreciated on CT or PET imaging, and the endoscopist should describe the size as well as the location (e.g., distance from incisors, relationship to adjacent mediastinal structures, etc.) of these, further facilitating accurate definition of potentially involved lymph nodes, either adjacent to or well removed from the primary tumor itself. In addition, radiographic areas of lymphadenopathy should similarly be included in the GTV.
Although the utility of PET in esophageal cancer staging primarily lies in its ability to detect distant metastases not fully appreciated on CT imaging (and thereby alter treatment approach of these patients),100,101 diagnostic PET/CT has more recently been integrated into radiation planning of esophageal cancer patients and definition of GTV. Generally, gross disease as defined on PET is characterized by a standard uptake value of >2 to 2.5.102 Generally speaking, PET-avid nodes should be included within the GTV volume. In a study from Fox Chase Cancer Center, the mean GTV length as determined by PET/CT closely correlated with endoscopy findings.59 Another study from Australian investigators reported that CT-alone–based definition of GTV excluded PET-avid disease in a majority of patients, resulting in a potential geographic miss of disease in a significant minority of patients.103 Similarly, the fusion of CT-PET has been shown to prompt GTV and planning target volume (PTV) modification in a majority of patients.104 Recent analysis of PET/CT-based (as compared to CT alone) radiotherapy planning of esophageal cancer patients indicated a reduction of intraobserver and interobserver variability in GTV delineation.105
Finally, although the routine use of fluoroscopic barium swallow in esophageal cancer has decreased, this study may still be useful to the radiation oncologist for field design. A study by Chinese investigators suggested that, as compared to CT, endoscopy with barium swallow more accurately defines the true length of middle and lower esophageal tumors, although CT appeared to better determine this for tumors located at the GE junction.106
In summary, accurate definition of primary and nodal gross disease is paramount in radiation esophageal cancer planning. It is important to rely on all diagnostic studies, including barium swallow (when available), EGD, EUS, and CT, as well as PET scan.
The identification of potential direct and nodal pathways for spread of subclinical disease (i.e., CTV definition) in esophageal cancer is also of paramount importance. These areas vary significantly, depending on site of origin of disease, making esophageal cancer planning somewhat complex. In terms of direct disease extension along the esophagus itself, varying reports have assessed the histologic findings at primary esophagectomy to determine subclinical extent of disease. One prospective analysis of 66 resection specimens showed that placement of a 3-cm margin proximally and distally on the primary tumor would cover microscopic disease extension in 94% of squamous cell carcinomas. Similarly, in GE junctional carcinomas, a 3-cm proximal margin included subclinical disease extension in 100% of patients, and 5 cm distally covered 94% of subclinical spread.106
As described previously, the esophagus is characterized by a rich submucosal network of lymphatics that facilitates early lymph node spread of disease, even for superficial esophageal cancers. The appropriate cranial and caudal margins remain a matter of debate in the treatment of esophageal cancer. Historically, very large fields were treated, encompassing potential pathways and lymphatic spread from the thoracic inlet down to the celiac axis region; however, such fields are potentially fraught with treatment-related toxicity and may be difficult for patients to tolerate, particularly in the context of concurrent chemotherapy delivery. Most contemporary radiation trials used margins of 3 to 5 cm cranially and caudally on the GTV, along with an approximate 2-cm radial margin. With disease located at or above the carina (or middle/upper one-third of the esophagus in some instances), many of these trials recommended fields inclusive of the supraclavicular lymph node basins, whereas celiac axis nodal basin coverage was recommended for disease of the distal esophagus.107 Further description of lymph node basin coverage follows.
Field Design
Historically, the treatment of very proximal (cervical) esophageal cancers has been challenging. Because of the changing contour from the neck to the thoracic inlet, treatment of lesions in the upper one-third of the esophagus may present a difficult technical problem. Lesions in the upper cervical or postcricoid esophagus are treated from the laryngopharynx to the carina, depending on extent of disease. Supraclavicular and superior mediastinal nodes are irradiated electively. Using older/conventional methods, this was achieved with lateral parallel opposed or oblique portals to the primary tumor and a single anterior field for the supraclavicular and superior mediastinal nodes.93 Another historical technique treated lesions in this region by means of a four-field box approach, using a wax bolus to build up the lack of tissue above the shoulders, acting as a compensator. A high-energy beam (>15 MV) is used, and both sides of the neck are treated prophylactically. Other methods of treating lesions at the thoracic inlet include 140-degree arc rotations, anterior wedged pairs, and three- or four-field techniques using posterior oblique portals combined with a single anterior portal or anteroposterior–posteroanterior (AP/PA) fields.93 Using three-dimensional (3D) approaches, varying techniques have been implemented, including treatment of the primary tumor and lymph nodes using an AP/PA approach to 39.6 to 41.4 Gy at 1.8 Gy per fraction, followed by a left or right opposed oblique pair to bring the total dose to 50.4 Gy, thereby limiting the spinal cord dose. This technique will generally exclude the supraclavicular fossa, and a separate electron field is often added, treating to a depth of 2 to 3 cm, depending upon individual anatomy. More recently, however, intensity-modulated radiation therapy (IMRT)–based planning has facilitated the treatment of upper esophageal lesions and is our preferred method for treating these tumors (Fig. 53.9) Strict normal tissue constraints, including normal lung and spinal cord, are important considerations in using these techniques (discussed later).
Based on the previously described and other pathologic patterns of spread data in squamous cell carcinoma of the esophagus, general guidelines in terms of field design can be made as follows: For cervical and upper thoracic squamous cell carcinoma, the CTV should generally include nodal basins extending from the lower cervical and supraclavicular region superiorly to the subcarinal lymph node basin inferiorly, inclusive of the upper paraesophageal lymph nodes (Fig. 53.9). For lower esophageal squamous cell carcinomas, lymph node basins from the subcarinal region superiorly to the left gastric and common hepatic artery/celiac lymph nodal basins inferiorly should generally be included (described further later in this chapter). For tumors of the middle esophagus, we recommend individual field design according to clinical scenarios, with a more complete coverage of paraesophageal mediastinal lymph nodes, particularly in patients with a good performance status (Fig. 53.10).37 These are general guidelines, and all plans should be individualized based on available imaging and endoscopic findings.
In field design, potential nodal involvement (and therefore target volumes) on nodal size is problematic, given that some reports demonstrated that <15% of metastatic nodes are >1 cm and that average size differences between involved and uninvolved nodes are frequently not significantly different.108 In addition, fluorodeoxyglucose-PET scanning has an estimated sensitivity of only 67% of detecting nodal metastases.8 Even endoscopic ultrasound, generally considered the most sensitive test for detecting lymph node metastases, is only able to detect such disease in approximately 75% of patients.109 Therefore, it is not appropriate to rely exclusively on varying imaging modalities to define areas of subclinical spread for esophageal cancer, realizing that patterns-of-spread data are important in determining radiation field design.
The design of radiation fields for the treatment of adenocarcinoma of the esophagus is similar to that of lower thoracic squamous cell carcinomas but deserves special mention. Periesophageal lymph nodes are generally included in all patients. Given that lymph node involvement is clearly associated with depth of tumor penetration (T stage) and the fact that most patients in the United States and Europe presenting with GE junctional carcinoma will have more advanced disease, inclusion of celiac lymph basins for adenocarcinoma of the distal esophagus/GE junction is usually indicated. Based on the previously described patterns of spread data from Erlangen and others, specific considerations include the following: Lymph vascular invasion is highly predictive of nodal spread. Proximal extension of tumors (particularly beyond the Z-line into the distal esophagus for type II and III tumors) predicts an increasing incidence of paraesophageal lymph node involvement. Based on an estimated nodal incidence cutoff of 20% for inclusion, specific considerations include the following: (a) The lower paraesophageal, paracardial, lesser curvature, and left gastric artery nodes should be included in the CTV. (b) The presence of lymph vascular invasion predicts a nodal positivity rate of >20% in the left and right gastroepiploic, greater curvature, celiac trunk, and splenic hilar regions. (c) In T3/4 disease, the gastroepiploic, greater curvature, celiac trunk, splenic hilar, splenic artery, and common hepatic artery should be included. (d) High-grade tumors should also include the left gastroepiploic, greater curvature, and celiac trunk nodes in CT design. (e) Larger and more deeply penetrating tumors should also include the splenic artery and splenic hilar nodes, as well as those along the greater curvature. (f ) Tumors extending above the diaphragm and those extending >1.5 cm beyond the Z-line should include the mid paraesophageal nodes, treating up to the carina. Of note, significant involvement of the distal esophagus by GE junctional tumors (>1.5 cm beyond the Z-line) should lead to inclusion of not only lower, but also middle paraesophageal nodes based on patterns of spread. However, treating these more extensive fields must be weighed against potential side effects of increased normal-tissue irradiation. In summary, middle and lower paraesophageal nodes should be included in patients with T2-T4 type I and T2-T4 type II tumors extending >1.5 cm above the Z-line and T3-T4 type II patients. The splenic hilar and artery nodes are considered “spareable” in T2 tumors, notably type I.42
FIGURE 53.9. A–C: Examples of planning target volume (PTV) in a 62-year-old woman with a cT3 N1 squamous cell carcinoma of cervical esophagus. Gross tumor volume (GTV), blue volume; PTV, red volume. D: Three-dimensional reconstruction of PTV from above the patient. Carina, yellow; GTV, dark blue; lungs, light blue; PTV, red; spinal cord, brown. E: Isodose curves of this patient treated with a nine-field intensity-modulated radiation therapy plan. Note that beams are primarily anteriorly oriented. (Courtesy of Rodney Hood, CMD.)





