Esophageal and Gastric Canc
The incidence of esophageal cancer has steadily increased while the incidence of gastric cancer has decreased in the United States for over a half century. Over the past several decades, the incidence of tumors in the distal esophagus/gastroesophageal junction and cardia is rising. The highest rates of esophageal cancer are found in East Asia, Eastern Africa, and Southern Africa. Over half of all gastric cancers occur in developing countries with the highest incidence in East Asia, South America (Andes Region), and Eastern Europe. Following migration to areas of lower risk, subsequent generations experience a risk approaching that of the surrounding population, implicating an important role for environmental factors on the development of gastric cancer. In the United States, there were estimated to be 17,990 new cases of esophageal cancer and 15,210 deaths in 2013. The estimated new cases and deaths of gastric cancer were 21,600 and 10,990, respectively (1). Advances in prevention, early detection, aggressive surgery, the use of adjuvant therapy, and more effective antineoplastic agents will hopefully reduce the incidence and improve survival.
PATHOLOGY
• Esophageal
• Squamous cell carcinoma: decreasing incidence and may arise throughout the esophagus
• Adenocarcinoma: increasing incidence and arises in the distal esophagus and gastroesophageal junction
• Gastric
• Intestinal type (expanding): characterized by the formation of distinct glands, and typically involves the cardia, corpus, or antrum. It is often associated with multifocal (atrophic) gastritis and intestinal metaplasia of the antrum, as well as pernicious anemia, older age, male sex, and various environmental factors, including Helicobacter pylori. There has been a dramatic decrease in the incidence of this form of gastric cancer in developing countries.
• Diffuse type (infiltrative): often presents as linitis plastica. It is characterized by poorly organized clusters or signet-ring cells (mucin containing). They often arise in the corpus and affect a generally young population. There is a propensity for these tumors to develop in patients with superficial gastritis related to H. pylori without atrophy or metaplasia, as well as those who have the type A blood group. Familial clusters are common. These tumors generally tend to be more aggressive than the intestinal type.
RISK FACTORS
• Esophageal
• Squamous cell: cigarette smoking, alcohol, achalasia, tylosis, caustic stricture (i.e., lye), prior radiation.
• Adenocarcinoma: Barrett’s esophagus due to GERD, smoking, obesity, higher socioeconomic class, Caucasian, male.
• Gastric
• Diets rich in salty or smoked foods, nitroso compounds, low in vegetable and antioxidants.
• H. pylori infection, which is dependent on genotype and host factors (polymorphisms).
• Smoking increases the risk by about 1.5-fold.
• Atrophic gastritis increases the risk by nearly sixfold.
• Prior gastric surgery with the highest risk at 15-20 years. The risk is greater following Billroth II than Billroth I anastomosis.
• Ionizing radiation was associated with a relative risk of 3.7 in survivors of the Japanese atomic bomb.
• Blood group A is associated with a 20% higher incidence.
• Low-socioeconomic group results in an increase in distal cancers, whereas high-socioeconomic group increases the risk of proximal cancers.
• Epstein-Barr virus associated gastric cancer is related to DNA methylation of promoter genes of various cancer-associated genes. They may have a more favorable prognosis.
• Several familial syndromes have been associated with a predisposition to gastric cancer: Lynch syndrome, E-cadherin (CDH1) mutation (diffuse type), familial adenomatous polyposis, and Peutz-Jeghers syndrome.
SIGNS AND SYMPTOMS
Progressive dysphagia leading to weight loss is the most common symptom of esophageal cancer. Abdominal pain and weight loss are common presenting complaints in gastric cancer. Nausea and vomiting are more commonly seen with distal gastric cancers, whereas early satiety is more common with linitis plastica tumors. Gastric cancers may bleed, leading to hematemesis, melena, and anemia. Malignant ascites, resulting in increased abdominal girth, are more commonly seen in patients with linitis plastica.
DIAGNOSIS AND STAGING EVALUATION
• Physical examination may be remarkable for cachexia, abdominal distension, hepatomegaly in the case of liver metastases, and lymphadenopathy.
• Upper GI series may demonstrate a stricture in the esophagus, a filling defect along the gastric wall, or decreased distensibility of the stomach due to a linitis plastica tumor.
• Esophagogastroduodenoscopy is the mainstay of diagnosis. Deep biopsies are often necessary if linitis plastica of the stomach is suspected as the tumor tends to infiltrate the submucosa. A single biopsy of a malignant ulcer has a 70% sensitivity rate of diagnosis and seven biopsies increase the sensitivity in gastric cancer to 98%.
• Endoscopic ultrasound aids in determining the depth of invasion, which may be important for determination of the need for neoadjuvant therapy or clinical trial considerations.
• CT scan of the chest, abdomen, and pelvis is important for the identification of metastatic disease.
• Bone scan is typically reserved for patients with symptoms suggesting osseous metastases.
• PET scan is commonly used to detect metastatic disease for esophageal cancer. The role in gastric cancer has not entirely been defined.
• Laparoscopy may detect occult peritoneal or hepatic metastases too small to be appreciated by CT scan and is often considered if imaging studies raise concern for peritoneal disease or in the case of bulky adenopathy.
• Tumor markers including CEA and CA 19-9 are sometimes helpful in monitoring patients with adenocarcinoma but are frequently not elevated.
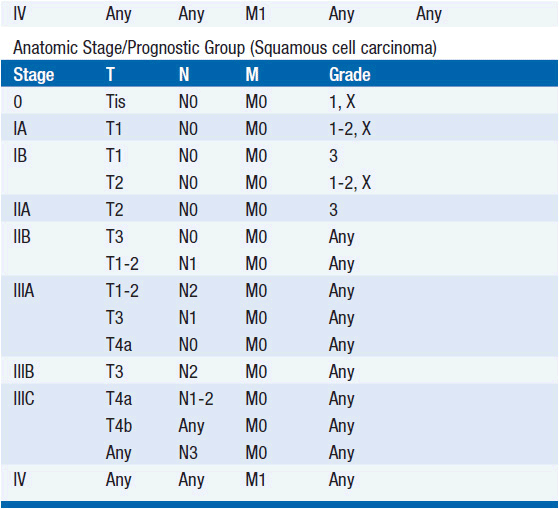
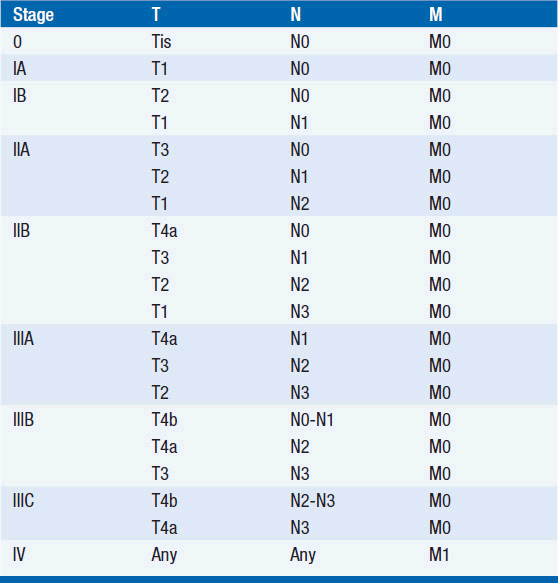
STAGING (AJCC)
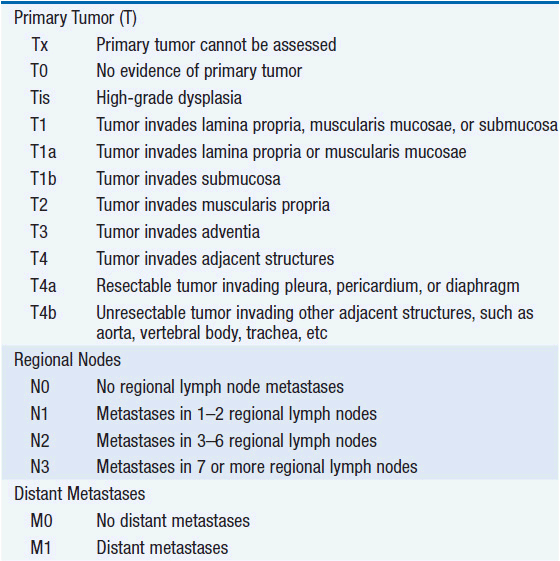
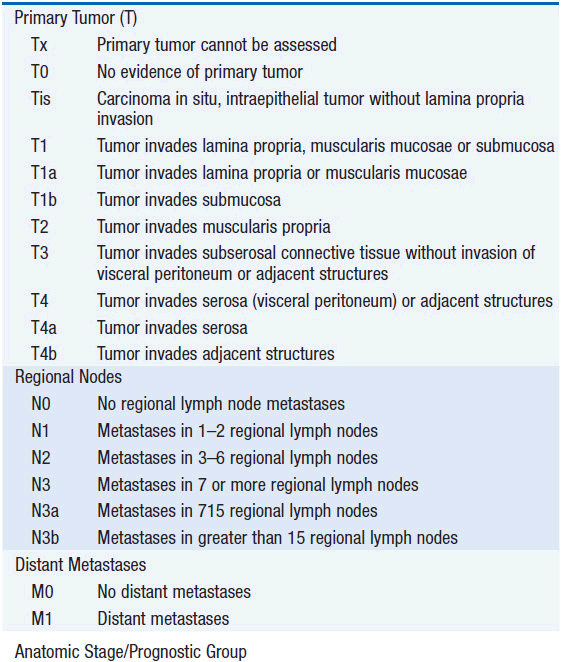
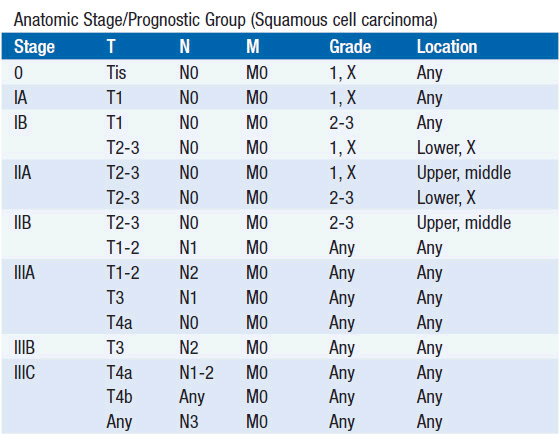
The American Joint Committee on Cancer is the system used for esophageal and gastric cancer staging in most countries (2). The seventh edition utilizes a separate staging system for squamous cell and adenocarcinoma of the esophagus and also incorporates grade. T stage is based on depth of invasion and N stage is based on total number of involved lymph nodes rather than location (unless distant lymph node). See Tables 44-1 and 44-2. The Siewert-Stein classification is based on the location of a tumor about the GE junction (3). A type I tumor has its center within 1-5 cm above the anatomic GE junction. A type 2 tumor is centered between 1 cm above and 2 cm below the GE junction. A type 3 tumor is centered 2-5 cm below the GE junction, in the cardia. Tumors arising in the cardia, within 5 cm, and invading the esophagus are staged according to esophageal cancer, whereas tumors located in the cardia of the stomach and not invading the GE junction are staged as gastric cancer.
TREATMENT OF LOCALiZED DiSEASE
 SURGICAL AND INVASIVE APPROACHES
SURGICAL AND INVASIVE APPROACHES
Esophageal Cancer
Less than half of all patients presenting with a new diagnosis of esophageal cancer have disease that is potentially resectable. The type of intervention offered to these patients may depend upon the depth of invasion of the tumor, location, comorbidities, and the preference of the surgeon. In patients with high-grade dysplasia or superficial cancers (i.e., T1a), particularly in those who are felt to be at high risk for esophagectomy, radio-frequency ablation, argon plasma coagulation, or photodynamic therapy (PDT) may be considered.
• Transhiatal esophagectomy: involves an upper midline laparotomy and left neck incision. A cervical anastomosis is created by a gastric pull-up. There is a limited ability to do thoracic lymphadenectomy.
• Ivor-Lewis transthoracic esophagectomy: involves a right thoracotomy and a laparotomy with an intrathoracic anastomosis. This procedure may be modified with a left thoracotomy for GE junction tumors.
Gastric Cancer
Approximately 50% of gastric cancers present with locoregional disease. The 5-year survival of patients with gastric cancer is only 15%–20%, but in those with disease only involving the stomach, the 5-year survival is 50%. Survival falls to about 20% once the regional nodes are involved by tumor. Curative surgery typically consists of a subtotal or total gastrectomy. Although the incidence of gastric cancer has been decreasing, the incidence of proximal gastric cancer and cancer of the gastroesophageal (GE) junction have dramatically increased. These more proximal tumors are associated with a poorer prognosis than their distal counterparts.
• Distal tumors: tumors arising in the distal two-thirds of the stomach are typically amenable to subtotal gastrectomy.
• Proximal tumors are usually managed with a total gastrectomy.
• Linitis plastica tumors are diffusely infiltrative, more commonly seen in the young, and typically metastatic at the time of diagnosis. If surgery is indicated, a total gastrectomy is the preferred operation.
LYMPH NODE DISSECTION
The magnitude of lymph node dissection required has remained a contentious area of debate in the surgical management of gastric cancer. For many years, the Japanese have advocated for an extended lymph node dissection in which the lymph nodes of the perigastric (D1 dissection), in addition to the lymph nodes of the hepatic, left gastric, celiac, splenic arteries, and splenic hilum (D2 dissection), as well as the nodes in the porta hepatis and periaortic areas (D3 dissection), are removed. Proponents of the extended lymph node dissection argue that patients will be more accurately staged, leading to a better stage-related survival. Such aggressive dissections may require a distal pancreatectomy and splenectomy, leading to considerable additional morbidity. Randomized trials have failed to demonstrate an improvement in survival for the more aggressive D3 dissections. In a 15-year follow-up of the Dutch D1D2 trial, D2 lymphadenectomy was associated with a lower locoregional recurrence and gastric cancer-related death rate than D1 surgery (4). The D2 dissection is associated with higher morbidity and mortality. As a result, there has been a move away from performing distal pancreatectomy and splenectomy. At least 15 lymph nodes should be removed.
ROLE OF DEFINITIVE CHEMORADIATION
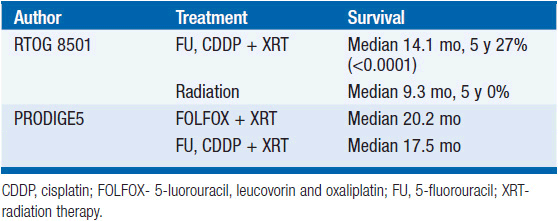
Tumors arising in the cervical esophagus are generally not amenable to surgical resection and are primarily treated with definitive chemoradiation (Table 44-3). The Radiation Therapy Oncology Group (RTOG)-8501 study demonstrated an improvement in median survival for those who received chemoradiation with 5-fluorouracil (5-FU), cisplatin, and 50 Gy compared to 64 Gy of radiation alone (5). The 5-year survival of the chemoradiation group was 27% compared to 0% in the radiation alone group. The PRODIGE 5/ACCORD17 Trial showed that FOLFOX (5-FU, leucovorin, and oxaliplatin) with radiation was equally efficacious and less toxic than 5-FU, cisplatin, and radiation (6). Surgical series utilizing neoadjuvant chemoradiation have demonstrated pathologic response rates of nearly 22%–40%, making chemoradiation a legitimate alternative to surgical resection (7–9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree