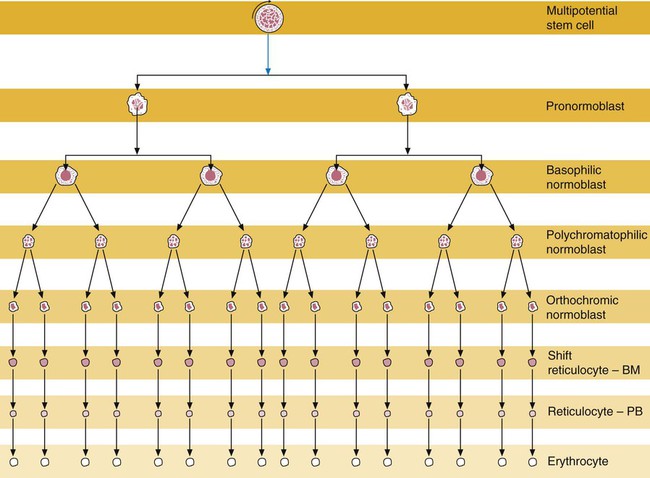
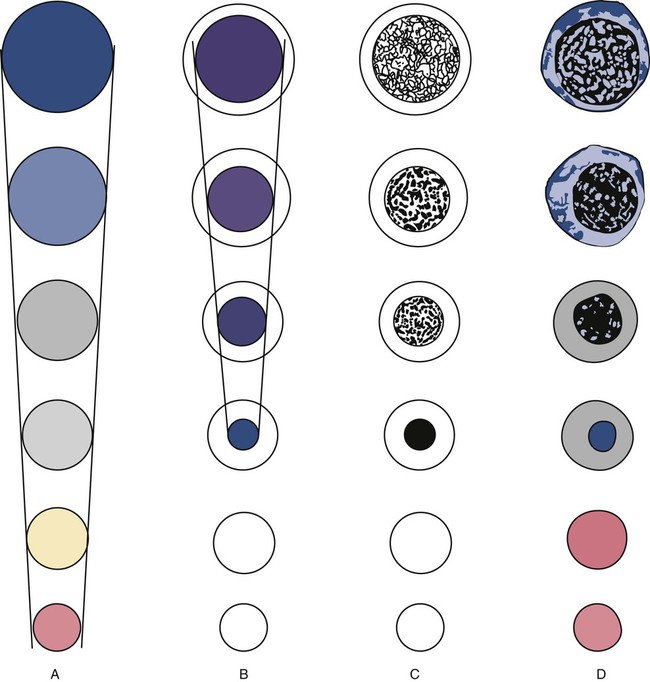
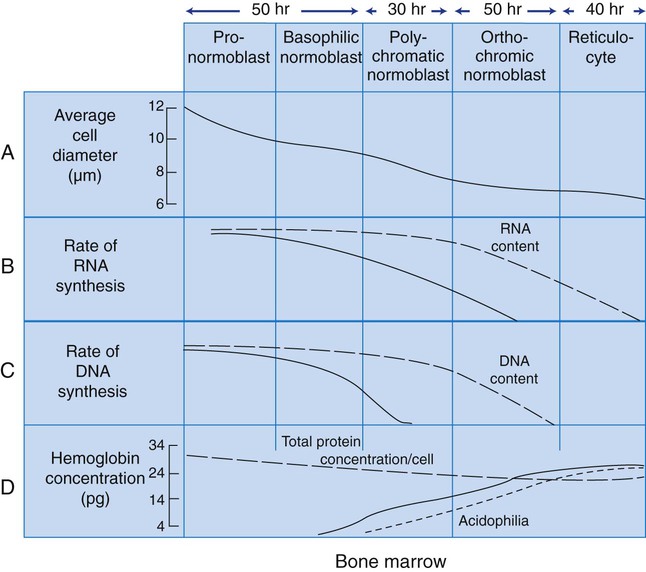
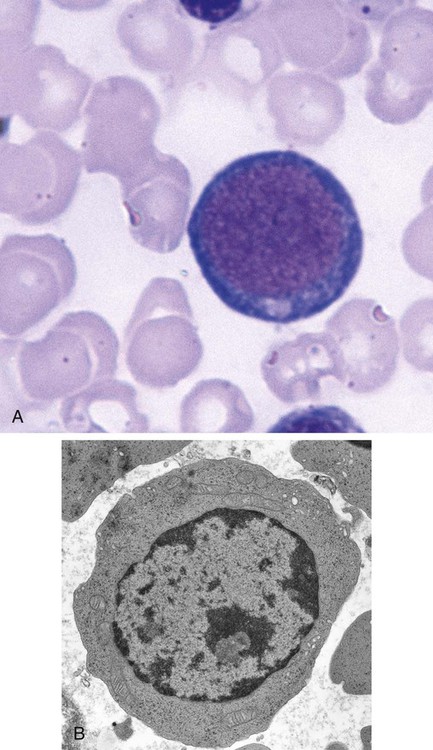
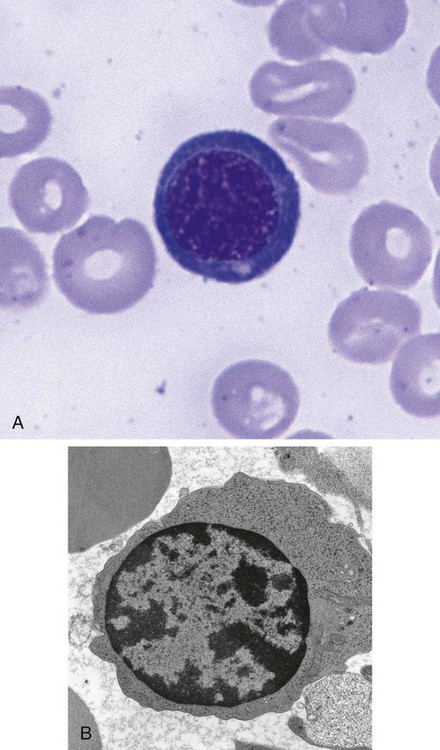
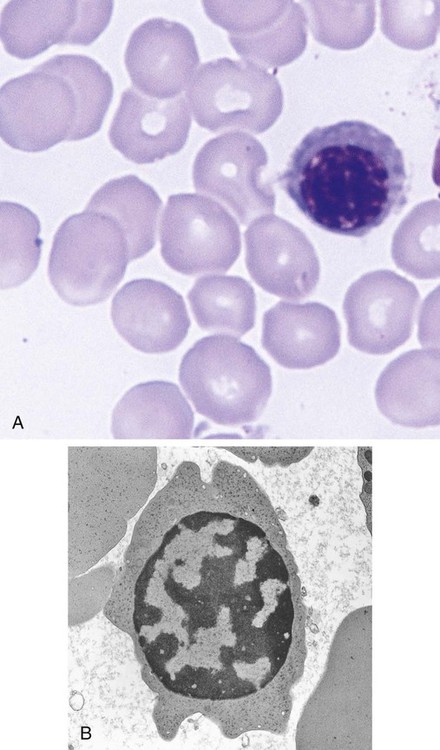
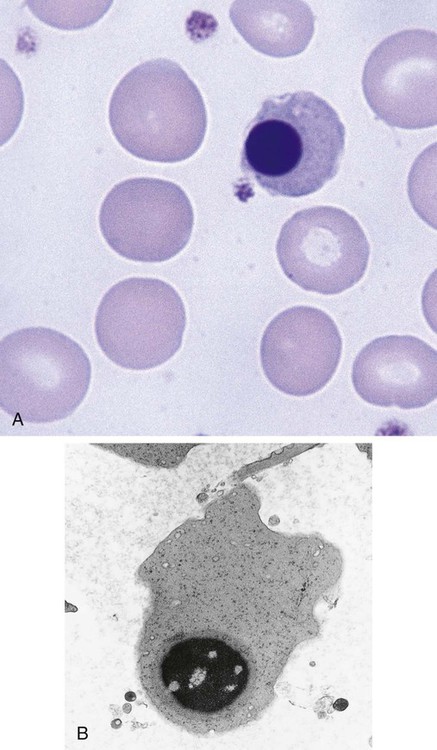
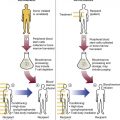
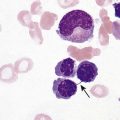
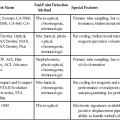
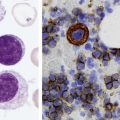
After completion of this chapter, the reader will be able to: 1. List and describe the erythroid precursors in order of maturity, including the morphologic characteristics, cellular activities, normal location, and length of time in the stage for each. 2. Correlate the erythroblast, normoblast, and rubriblast nomenclatures for red blood cell (RBC) stages. 3. Name the stage of erythroid development when given a written description of the morphology of a cell in a Wright-stained bone marrow smear. 4. List and compare the cellular organelles of immature and mature erythrocytes and describe their specific functions. 5. Name the erythrocyte progenitors and distinguish them from precursors. 6. Explain the nucleus-to-cytoplasm (N : C) ratio. Describe the appearance of a cell when given the N : C ratio or estimate the N : C ratio from the appearance of a cell. 7. Explain how reticulocytes can be recognized in a Wright-stained peripheral blood film. 8. Define and differentiate the terms polychromasia, diffuse basophilia, punctate basophilia, and basophilic stippling. 9. Discuss the differences between the reticulum of reticulocytes and punctate basophilic stippling in composition and conditions for viewing. 10. Define and differentiate erythron and RBC mass. 11. Explain how hypoxia stimulates RBC production. 12. Describe the general chemical composition of erythropoietin (EPO) and name the site of production. 13. Discuss the various mechanisms by which EPO contributes to erythropoiesis. 14. Define and explain apoptosis resulting from Fas/FasL interactions and how this regulatory mechanism applies to erythropoiesis. 15. Explain the effect of bcl-xL and the general mechanism by which it is stimulated in red blood cell progenitors. 16. Describe the features of the bone marrow that contribute to establishing the microenvironment necessary for the proliferation of RBCs, including location and arrangement relative to other cells, with particular emphasis on the role of fibronectin. 17. Discuss the role of macrophages in RBC development. 18. Explain how RBCs enter the bloodstream and how premature entry is prevented and, when appropriate, promoted. 19. Describe the characteristics of senescent RBCs and explain why RBCs age. 20. Explain and differentiate the two normal mechanisms of erythrocyte destruction, including location and process. Three nomenclatures are used for naming the erythroid precursors (Table 8-1). The erythroblast terminology is used primarily in Europe. Like the normoblastic terminology used more often in the United States, it has the advantage of being descriptive of the appearance of the cells. Some prefer the rubriblast terminology because it parallels the nomenclature used for white blood cell development. Normoblastic terminology is used in this chapter. TABLE 8-1 Three Erythroid Precursor Nomenclature Systems As described in Chapter 7, the morphologically identifiable erythrocyte precursors develop from two functionally identifiable progenitors, burst-forming unit–erythroid (BFU-E) and colony-forming unit–erythroid (CFU-E), committed to the erythroid cell line. Estimates of time spent at each stage suggest that it takes about 1 week for the BFU-E to mature to the CFU-E and another week for the CFU-E to become a pronormoblast,1 which is the first morphologically identifiable RBC precursor. While at the CFU-E stage, the cell completes approximately three to five divisions before maturing further.1 As seen later, it takes approximately another 6 days for the precursors to become mature cells ready to enter the circulation, so approximately 18 to 21 days are required to produce a mature RBC from the BFU-E. Normoblastic proliferation, similar to the proliferation of other cell lines, is a process encompassing replication (i.e., division) to increase cell numbers and development from immature to mature cell stages (Figure 8-1). The earliest morphologically recognizable erythrocyte precursor, the pronormoblast, is derived via the BFU-E and CFU-E from the multipotential stem cells as discussed in Chapter 7. The pronormoblast is able to divide, with each daughter cell maturing to the next stage of development, the basophilic normoblast. Each of these cells can divide, with each of its daughter cells maturing to the next stage, the polychromatophilic normoblast. Each of these cells also can divide and mature. In the erythrocyte cell line, there are typically three and occasionally as many as five divisions2 with subsequent nuclear and cytoplasmic maturation of the daughter cells, so that from a single pronormoblast, eight mature RBCs usually result. The conditions under which the number of divisions can be increased or reduced are discussed later. Morphologic identification of blood cells depends on a well-stained peripheral blood film or bone marrow smear (see Chapter 16). In hematology, a modified Romanowsky stain, such as Wright or Wright-Giemsa, is commonly used. The descriptions that follow are based on the use of these types of stains. The stage of maturation of any blood cell is determined by careful examination of the nucleus and the cytoplasm. The qualities of greatest importance in identification of RBCs are the nuclear chromatin pattern (texture, density, homogeneity), nuclear diameter, nucleus/cytoplasm (N : C) ratio (Box 8-1), presence or absence of nucleoli, and cytoplasmic color. As RBCs mature, several general trends affect their appearance. Figure 8-2 graphically represents these trends. 1. The overall diameter of the cell decreases. 2. The diameter of the nucleus decreases more rapidly than does the size of the cell. As a result, the N : C ratio also decreases. 3. The nuclear chromatin pattern becomes coarser, clumped, and condensed. The nuclear chromatin of RBCs is inherently coarser than that of myeloid precursors, as if it is made of rope rather than yarn. It becomes even coarser and more clumped as the cell matures, developing a raspberry-like appearance, in which the dark staining of the chromatin is distinct from the almost white appearance of the parachromatin. This chromatin/parachromatin distinction is more dramatic than in myeloid cells. Ultimately, the nucleus becomes quite condensed, with no parachromatin evident at all, and the nucleus is said to be pyknotic. 4. Nucleoli disappear. Nucleoli represent areas where the ribosomes are formed and are seen early as cells begin actively synthesizing proteins. As RBCs mature, the nucleoli disappear, which precedes the ultimate cessation of protein synthesis. 5. The cytoplasm changes from blue to gray to pink. Blueness or basophilia is due to acidic components that attract the basic stain, such as methylene blue. The degree of cytoplasmic basophilia correlates with the amount of ribosomal RNA. These organelles decline over the life of the developing RBC, and the blueness fades. Pinkness or eosinophilia is due to more basic components that attract the acid stain eosin. Eosinophilia of erythrocyte cytoplasm correlates with the accumulation of hemoglobin as the cell matures. Thus the cell starts out being active in protein production on the ribosomes that make the cytoplasm quite basophilic, transitions through a period in which the red of hemoglobin begins to mix with that blue, and ultimately ends with a thoroughly pink-red color when the ribosomes are gone and only hemoglobin remains. Table 8-2 lists the stages of RBC development in order and provides a convenient comparison. The listing makes it appear that these stages are clearly distinct and easily identifiable. Similar to the maturing of children into adults, however, the process of cell maturation is a gradual process, with changes occurring in a generally predictable sequence but with some variation for each individual. The identification of a given cell’s stage depends on the preponderance of characteristics, although the cell may not possess all the features of the archetypal descriptions that follow. Essential features of each stage are in italics in the following descriptions. The cellular functions described subsequently also are summarized in Figure 8-3. TABLE 8-2 Normoblastic Series: Summary of Stage Morphology
Erythrocyte Production and Destruction
Normoblastic Maturation
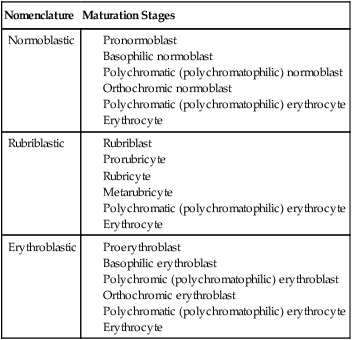
Terminology
Nomenclature
Maturation Stages
Normoblastic
Rubriblastic
Erythroblastic
Maturation Process
Erythroid Progenitors
Erythroid Precursors
Criteria Used in Identification of the Erythroid Precursors
Maturation Sequence
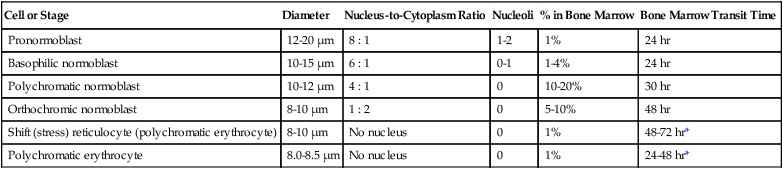
Cell or Stage
Diameter
Nucleus-to-Cytoplasm Ratio
Nucleoli
% in Bone Marrow
Bone Marrow Transit Time
Pronormoblast
12-20 µm
8 : 1
1-2
1%
24 hr
Basophilic normoblast
10-15 µm
6 : 1
0-1
1-4%
24 hr
Polychromatic normoblast
10-12 µm
4 : 1
0
10-20%
30 hr
Orthochromic normoblast
8-10 µm
1 : 2
0
5-10%
48 hr
Shift (stress) reticulocyte (polychromatic erythrocyte)
8-10 µm
No nucleus
0
1%
48-72 hr*
Polychromatic erythrocyte
8.0-8.5 µm
No nucleus
0
1%
24-48 hr*