INTRODUCTION
SUMMARY
Blood group antigens are structures on the outer surface of human red blood cells (RBCs) that can be recognized by the immune system of individuals who lack that particular structure. Identification of RBC antigens and antibodies has been the basis of pretransfusion compatibility testing and the safe transfusion practices used today and also can provide insights into understanding the etiology of hemolytic disease of the fetus and the newborn. Biochemical and molecular studies have led to definition of the biologic functions of molecules expressing blood group antigens. These molecules play a critical role in susceptibility to infection by malarial parasites, some viruses, and bacteria. Alteration of RBC antigen expression is associated with many molecular backgrounds and some play a role in the clinical manifestations of certain diseases. Erythrocytes, far from being inert containers of hemoglobin, are active in a variety of physiologic processes.
Acronyms and Abbreviations
AET, 2-aminoethylisothiouronium bromide; CD, cluster of differentiation; DTT, dithiothreitol; GPA, glycophorin A; GPB, glycophorin B; GPC, glycophorin C; GPD, glycophorin D; GPI, glycosylphosphatidylinositol; HDFN, hemolytic disease of the fetus and newborn; HEMPAS, hereditary erythroblastic multinuclearity with a positive acidified serum test; Ig, immunoglobulin; ISBT, International Society of Blood Transfusion; LAD, leukocyte adhesion deficiency; 2-ME, 2-mercaptoethanol; PNH, paroxysmal nocturnal hemoglobinuria; RBC, red blood cell.
DEFINITIONS AND HISTORY
A blood group system consists of a group of antigens encoded by alleles at a single gene locus or at gene loci so closely linked that crossing over does not occur or is very rare. An antigen collection consists of antigens that are phenotypically, biochemically, or genetically related, but the genes encoding them have not been identified.1 Placement of a blood group antigen into a system or collection begins with the discovery of an antibody, usually in the serum of a multiparous woman or a multiply transfused recipient, with a unique pattern of reactivity. The antibody can be used to study basic biochemical properties of the corresponding antigen, to enable recognition of the pattern of inheritance of the antigen in families and in populations, to identify red blood cells (RBCs) that lack the antigen, and to search for an antithetical antigen. Identified characteristics, such as prevalence of positive reactions or sensitivity or resistance to specific enzymes, are compared to antigens in known systems and collections. A newly recognized antigen is also evaluated using biochemical and molecular genetic methods. Orphan antigens of low or high prevalence are placed in “holding tanks” until the gene that encodes them is established.
The majority of genes encoding blood group antigens have been cloned and sequenced,2 and the molecular bases of most blood group antigens have been determined.3,4,5,6 Details on the alleles associated with blood group antigens and phenotypes can be obtained from the National Center for Biotechnology Information (NCBI) “dbRBC” website: and from the International Society of Blood transfusion (ISBT) website: www.isbt-web.org.
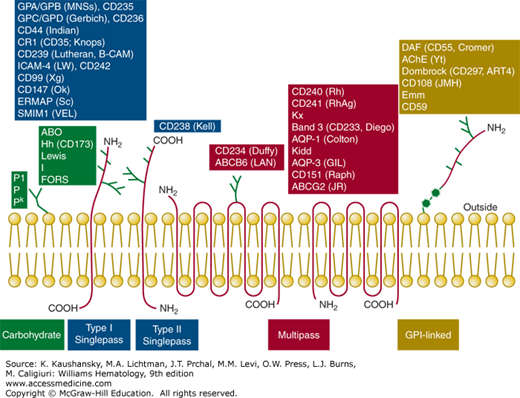
RBC antigens are inherited carbohydrate or protein structures located on the outside surface of the RBC membrane (Fig. 136–1). Although most of the protein blood group antigens are carried on integral transmembrane proteins (either single-pass type I or type II, or multipass; Fig. 136–1), a few are carried on glycosylphosphatidylinositol (GPI)-linked proteins or adsorbed from plasma. Some carbohydrate antigens are attached to proteins or lipids and some require a combination of a specific portion of protein and carbohydrate. Blood group antigens have revealed that certain transmembrane proteins interact with other transmembrane proteins (e.g., band 3 and glycophorin A [GPA]; Kell and Kx; Rh and RhAG), with lipids (e.g., Rh), or with proteins in the membrane skeleton (e.g., band 3 and ankyrin, glycophorin C [GPC], and protein 4.1 and p55). Many of the proteins carrying blood group antigens reside in the erythrocyte membrane as complexes.7,8,9,10 Many components carrying blood group antigens have been assigned cluster of differentiation (CD) numbers (Table 136–1). In human blood grouping, agglutination of RBCs usually serves as the detectable end point, but it can also be hemolysis.11 Our ability to detect and identify blood group antigens and antibodies has contributed significantly to current safe blood transfusion practice, reducing death from hemolytic disease of the fetus and newborn (HDFN) from 40 percent to less than 2 percent, and supporting patients receiving chemotherapy or organ transplantation.
| Conventional Name | ISBT Symbol (No.) | Chromosome Location | ISBT Gene Name (ISGN If Different) | Associated Antigens [Null Phenotype] | Function of RBC Membrane Component (CD No.) | Disease Association |
|---|---|---|---|---|---|---|
| BLOOD GROUP SYSTEMS | ||||||
| ABO | ABO (001) | 9q34.2 | ABO | A, B, A, B, A1 [group O] | Glycocalyx | Altered expression in some hematologic disorders |
| MNS | MNS (002) | 4q31.21 | MNS (GYPA, GYPB) | M, N, S, s, U, and 43 more [En(a–); U–; MkMk] | Binds microbe glycocalyx, complement regulation, chaperone for band 3 (CD235) | Decreased Plasmodium falciparum invasion, may be receptor for Escherichia coli |
| P1PK | P1PK (003) | 22q13.2 | P1 (A4GALT) | P1, Pk, NOR | Glycocalyx | |
| Rh | RH (004) | 1p36.11 | RHD, RHCE (RH) | D, C, E, c, e, and 49 more [Rhnull] | Possibly transports CO2 or NH3 (CD240) | Hemolytic anemia, hereditary stomatocytosis, hematologic malignancies |
| Lutheran | LU (005) | 19q13.32 | LU | Lua, Lub, Lu3, and 18 more [recessive Lu(a–b–)] | Binds laminin (CD239) | Increased expressions possibly involved in vasoocclusion in sickle cell disease |
| Kell | KEL (006) | 7q34 | KEL | K, k, Kpa, Kpb, Jsa, Jsb, and 29 more [K0 or Knull] | Cleaves big endothelin-3 to ET-3, a potent vasoconstrictor (CD238) | |
| Lewis | LE (007) | 19p13.3 | LE (FUT3) | Lea, Leb, and 4 more [Le(a–b–)] | Glycocalyx, Leb is receptor for Helicobacter pylori | Increased expression in fucosidosis, Lewis antibodies may be important in graft rejection |
| Duffy | FY (008) | 1q23.2 | FY (DARC) | Fya, Fyb, Fy3, Fy5, Fy6 [Fy(a–b–)] | Chemokine, Plasmodium vivax receptor (CD234) | Resistance to P. vivax invasion |
| Kidd | JK (009) | 18q12.3 | JK(HUT11, SLC14AI) | Jka, Jkb, Jk3 [Jk(a–b–)] | Urea transporter | Impaired urea transport, urine-concentrating defect |
| Diego | DI (010) | 17q21.31 | DI (SLC4A1; AE1) | Dia, Dib, Wra, Wrb, and 18 more | Anion exchanger (CD233), Band 3 cytoskeletal protein | Southeast Asian ovalocytosis, hereditary spherocytosis, renal tubular acidosis |
| Yt | YT(011) | 7q22.1 | Yt (ACHE) | Yta, Ytb | Acetylcholinesterase | Absent from PNH III RBCs |
| Xg | XG (012) | Xp22.33 | XG (XG, MIC2) | Xga, CD99 | Adhesion molecules (CD99) | |
| Scianna | SC (013) | 1p34.2 | SC (ERMAP) | Sc1, Sc2, Sc3, and 4 more [SC: –1, –2, –3] | Possible adhesion | |
| Dombrock | DO (014) | 12p12.3 | DO (ART4) | Doa, Dob, Gya, Hy, Joa and 3 more [Gy(a–)] | Enzymatic (CD297) | Absent from PNH III RBCs |
| Colton | CO (015) | 7p14.3 | CO (AQP1) | Coa, Cob, Co3, Co4 [Co(a–b–)] | Water transport | Monosomy 7, inability to maximally concentrate urine, congenital dyserythropoietic anemia |
| Landsteiner-Wiener | LW (016) | 19p13.2 | LW (ICAM4) | LWa, LWab, LWb [LW(a–b–)] | Binds CD11/CD18, ligand for integrins (CD242) | Depressed in pregnancy and some malignant diseases |
| Chido/Rogers | CH/RG (017) | 6p21.32 | C4A,C4B | CH1, RGl, and 7 more | Complement components | Certain phenotypes have increased susceptibility to certain autoimmune conditions and infections |
| H | H (018) | 19q13.33 | H (FUT1) | H [Bombay, Oh] | Glycocalyx (CD 173) | Decreased in some tumor cells, increased in hematopoietic stress |
| Kx | XK (019) | Xp21.1 | XK | Kx [McLeod] | Possible neurotransmitter, function in RBCs not known | Acanthocytosis, muscular dystrophy, hemolytic anemia; McLeod syndrome sometimes associated with CGD, peripheral neuropathy, cardiomyopathy seizures, a late-onset dementia, and behavioral changes |
| Gerbich | GE (020) | 2q14.3 | GE (GYPC) | Ge2, Ge3, Ge4, and 8 more [Leach phenotype] | Membrane attachment; interacts with 4.1R and p55 (CD236) | Hereditary elliptocytosis, hemolytic anemia, decreased 4.1R and p55 |
| Cromer | CROM (021) | 1q32.2 | CROM (CD55; DAF) | Cra, Tca, Tcb, Tcc, Dra, and 13 more [Inab phenotype] | Complement regulation, binds C3b, disassembles C3/C5 convertase (CD55) | Absent from PNH III RBCs, Dra is the receptor for uropathogenic E. coli |
| Knops | KN (022) | 1q32.2 | KN (CR1) | Kna, Knb, McCa, SIa, Yka, and 4 more [Helgeson phenotype] | Complement regulation, binds C3b and C4b, mediates phagocytosis (CD35) | Antigens depressed in certain autoimmune and malignant conditions |
| Indian | IN (023) | 11p13 | IN (CD44) | Ina, Inb, INFI, INJA | Binds hyaluronic acid, mediates adhesion of leukocytes (CD44) | Depressed in pregnancy, congenital dyserythropoietic anemia |
| Ok | OK (024) | 19p13.3 | OK (BSG) | Oka, OKGV, OKVM | Possible adhesion (CD147) | |
| Raph | RAPH (025) | 11p15.5 | MER2 (CD151) | MER2 [RAPHnull] | Adhesion molecule involved in kidney function (CD151) | Renal disease, associated with pretibial epidermolysis bullosa and sensorineural deafness |
| John Milton Hagen | JMH (026) | 15q24.1 | JMH (SEMA7A) | JMH, and 5 more | Adhesion molecule, function in RBCs not known (CD108) | Absent from PNH III RBCs |
| I | I (027) | 6p24.2 | GCNT2 | I [I–; i adult] | Glycocalyx | Congenital cataracts in Asians |
| GLOB | Globoside (028) | 3q26.1 | P (β3GALNT1) | P, PX2 [P–] | Glycocalyx | Receptor E. coli and parvovirus B19 |
| Gil | GIL (029) | 9p13.3 | GIL (AQP3) | GIL [GIL–] | Glycerol/water/urea transporter | |
| Rh-associated glycoprotein | RHAG (030) | 6p21.3 | RHAG | Duclos, and 3 more | Possibly transports CO2, or NH3 (CD241) | Hemolytic anemia, hereditary stomatocytosis |
| FORS | FORS (031) | 9q34.2 | GBGT1 | FORS1 | Glycocalyx | |
| JR | JR (032) | 4q22.1 | JR, ABCG2 | Jra [Jr(a–)] | ATP-dependent transporter | |
| Lan | LAN (033) | 2q36 | LAN, ABCB6 | Lan [Lan–] | ATP-dependent transporter | |
| Vel | VEL (034) | 1p36.32 | VEL, SMIM1 | Vel [Vel-] | CD59 | |
| CD59 | CD59 (035) | 1p13.33 | CD59 | CD59.1 [CD59-] | Complement regulation (CD59) | Absent from PNH III RBCs |
| Antigen Collections | ||||||
| Cost | COST (205) | — | — | Csa, Csb | ||
| Ii | I (207) | — | — | i | ||
| Er | ER (208) | — | — | Era, Erb, Er3 | ||
| GLOB (209) | — | — | LKE, | |||
| Unnamed: (210) | — | — | Lec, Led | |||
| MN CHO (213) | — | — | Hu, M1, Tm, Can, Sext, Sj | |||
| Low-incidence series | — (700) | — | — | 17 | ||
| High-incidence series | — (901) | — | — | Ata, Emm, AnWj, Sda, PEL, ABTI, MAM | ||
The naming of blood group antigens usually does not follow the classic convention wherein dominant traits are given capital letters and recessive traits are designated with lowercase letters. For example, in the ABO blood group system, A and B are codominant and the recessive O phenotype is encoded by a gene designated O, whereas in the MNS system the genes S and s are codominant. To standardize terminology used to describe RBC blood groups, the ISBT Working Party for Terminology for Red Cell Surface Antigens recommended using the traditional name for an antigen for verbal communication and a numerical system in computer databases (see Blood Group Terminology website at www.isbt-web.org). The working party has placed blood group antigens into four categories: (1) genetically discrete blood group systems; (2) serologically, biochemically, or genetically related antigens in blood group collections; (3) series of low-incidence antigens; and (4) series of high-incidence antigens. Each system and collection has been given a number and letter designation, and each antigen within the system is numbered sequentially in order of discovery. At the time of going to press (2015), 35 blood group systems and 6 antigen collections are defined (see Table 136–1; www.isbt-web.org).1,5,6,12,13,14 Over time, notations devised to describe blood group antigens have changed. A single letter (e.g., A, D, K), a symbol with a superscript (e.g., Fya, Jkb, Lua), a symbol with a number (e.g., Fy3, Lu4, K12), and three to four letters (e.g., Vel, JMH, ELO, FPPT) are all used, sometimes within the same blood group system. The ISBT Working Party name has changed to ISBT Working Party on Red Cell Immunogenetics and Blood Group Terminology, which reflects that DNA testing is now often used to predict a blood group.
BLOOD GROUP SYSTEMS
Tables 136–1 and 136–2 summarize the characteristics of common blood group antigens. The following sources provide more detail: Issitt and Anstee,5 Reid, Lomas-Francis and Olsson,6 Reid and Lomas-Francis,15 Mollison and colleagues,16 Daniels,4 and Fung and associates.11 In the interest of space, reviews or books are referenced in place of original reports.
| Blood Group (Year Reported) | Common Phenotypes | Frequency White/Black (%) | No. Antigen Copies on Adult RBC × 103 | Dosage (See Text) | Cord Cell Expression | Biochemistry | Antigen Distribution in Blood, Fluids, and Tissues | Comments |
|---|---|---|---|---|---|---|---|---|
| ABO (1900) | A B AB | 40/27 11/20 4/4 | AB: ~800–1000 | A/B: not evident | Weak: ~1/3 adult expression | Carbohydrate on types 1, 2, 3, and 4 precursor chains | RBC, lymphs, plts | Most significant antigens in transfusion and transplantation |
| H (1948) | O | 45/29 | H: ~1700 | H expression depends on ABO: O>A2>B> A2B>A1>A1B | Weak Main RBC carrier: bands 3 and 4.5 | Attached to lipids in plasma and protein in secretions | Plasma, secretions; broad tissue distribution; most epithelial/endothelial cells | Weak subgroups result from variant transferases |
| Rh (1939) | R1 Dce r ce R2 DcE R0 Dce r’ Ce r″ cE | 42/17 37/26 14/11 4/44 2/2 1/0 | D on R2R2: 15–33 R1R1: 14–19 R0r: 12–20 R1r: 9–14 c on cc: 70–85 | D: not evident C and c: yes E and e: yes | Normal adult | Multipass, nonglycosylated protein: 30–32 kDa; 417 aa C: serine 103/c: proline 103 E: proline 226/e: alanine 226 | Erythroid specific Possible cation transport Possible role in RBC membrane integrity | D most significant antigen after A and B Three causes for weak D expression (see text) Nulls: amorphic type and regulator type |
Rz DCE ry CE | <1 <1 | Cc: 37–53 e on ee: 18–24 Ee: 13–14 | Forms “Rh complex” with LW, GPB, and Rh-related glycoprotein (chromosome 6) | |||||
| Lewis (1946) | Le(a+b–) Le(a–b+) Le(a–b–) Le(a+b+) | 22/23 72/55 6/22 Rare in European populations; found in Australasian populations; 10 in Japanese | Lea: ~3 | Not evident | Weak: adult expression at age 2 years | Carbohydrate on type 1 precursor chains only Attached to lipids in plasma and protein in secretions | Plasma and secretion antigen; on RBC, lymphs, plts only by adsorption of plasma antigen | Le antigens depend on Le/Se interaction; Le/Se = Le(a–b+), ABH secretor; lele/sese = Le(a+b–), ABH nonsecretor; lele = Le(a–b–), Sese status not apparent Le(a–b+) express some Lea, do not make anti-Lea. Women test Le(a–b–) during pregnancy |
| I (1956) | l adult(↑↑I↓i) i cord (↓I↑i) i adult (↓I↑↑i) | Common Common <1:10,000 | I: ~500 | Not evident | Strong i; weak I. Adult expressions at age 2 years | Carbohydrate on ABH active chains; lipid on RBC; protein in plasma | Broad tissue distribution; RBCs, plts, lymphs, granules, monos; also in plasma, secretions (e.g., milk, saliva, urine) | I and i expression are inversely proportional but not products of alleles |
| P1PK and GLOB | ||||||||
P1 (1927) GLOB (1951) | P1: P2: p: Pk–P–P1– | 79/94 21/6 Rare | P1: ~500 Globoside: ~15,000 | Not evident, but inherited variations exist; e.g., P1 may be normal, strong, or weak | Weak: adult expression by 7 years | Carbohydrate on RBC and plasma glycolipids; not in secretions | RBC, lymphs, plts, monos, fibroblasts, uroepithelial cells | P1-like antigen is associated with pigeon and earthworm protein and parasitic infections |
MNS (M: 1927) (S: 1947) | M+N– M+N+ M–N+ S+s– S+s+ S–s+ S–s–U– | 28/26 50/44 22/30 11/3 44/28 45/69 0/<1 | GPA: ~800 GPB: ~200 | Yes | Normal adult | Single-pass sialoglycoprotein type 1 GPA: 43 kDa, 131 aa, carries MN GPB: 25 kDa, 72 aa, carries SsU; part of Rh complex | RBCs plus renal capillary epithelial/endothelium | GPA and GPB carry multiple antigens and many hybrids of GPA–GPB Can have absence of GPA, GPB, or both |
| Kell (1946) | K–k+ | 91/98 | Kell: 2–6 | Yes | Normal adult | Single-pass glycoprotein type II highly folded: 93kDa, 732 aa | Kell: RBC plus marrow and fetal liver tissue; not on brain, kidney, adult liver | System of high- and low-frequency antigens |
| Kpa/Jsa: (1957) | K+k+ K+k– Kp(a–b+) Kp(a+b+) Kp(a+b–) Js(a–b+) Js(a+b+) Js(a+b–) | 8.8/2 0.2/rare 97.7/100 2.3/rare Rare/0 100/80 Rare/19 0/1 | K/k Met 193 Thr Kpa/Kpb: Trp 281 Arg Jsa/Jsb: Pro 597 Leu | Kx: RBC plus skeletal/heart muscle, neurologic tissues | Common phenotype: k, Kpb, Jsb Kell antigen expression depends on both Kell and Xk genes Knull lacks Kell antigens, has Kx Kxnull lacks Kx, has poor Kell antigen expression (McLeod phenotype) Other causes of poor Kell expression: cis Kpa, Ge-, Kmod, autoantibody | |||
| Duffy (1950) | Fy(a+b–) Fy(a+b+) Fy(a–b+) Fy(a–b–) | 17/9 49/1 34/22 Rare/68 | Fya: 6–13 | Yes, but not always evident because of Fy gene | Normal: adult levels at 12 weeks | Multipass glycoprotein: 35–45 kDa, 338 aa Fya/Fyb Gly 42Asp | RBC plus brain, colon, lung, spleen, thyroid, thymus, kidney, endothelium; not in liver or placenta tissue | Fy(a–b–) blacks do not express Fyb on their RBC, but express it on other tissues and seldom make anti-Fyb |
| Kidd (1951) | Jk(a+b–) Jk(a+b+) Jk(a–b+) Jk(a–b–) | 28/57 49/34 23/9 <1% Polynesians | Jka: ~14 | Yes | Normal adult | Multipass protein: ~43 kDa, 391 aa 1 potential N-glycan Jka/Jkb: Asp284 Asn | RBC specific | Important cause of DHTR Nulls are unable to fully concentrate urine; dominant inhibitor In(Jk) has weak Jk antigen |
| Lutheran (1951) | Lu(a+b–) Lu(a+b+) Lu(a–b+) Lu(a–b–) | 0.15/– 7.5/0 92.3/– Very rare | Lub: 1.5–4 | Yes, but family variations exist | Weak: adult level at 15 years | Single-pass glycoprotein type I: 85 kDa, 597 aa 78 kDa 5 Ig superfamily domains: two variable, three constant B-CAM Lua/Lub: His77Arg | RBC plus brain, heart, kidney, lung, pancreas, placenta, skeletal muscle | System of high- and low-frequency antigens Dominant inhibitor In(Lu) and X-linked inhibitor (XS2) cause greatly reduced Lu expression First known autosomal linkage to Se |
The ABO blood group system was the first system described and remains the most significant in transfusion medicine. A mismatch of ABO may be fatal, whereas a mismatch of other blood groups initially mostly is harmless. This situation occurs because anti-A and anti-B antibodies usually are present in the blood of adults lacking the corresponding antigen. These antibodies are stimulated by the ubiquitous distribution of the antigen that forms part of the membrane structure of many bacteria, plants, and animals. For this reason, all donor blood for transfusion is tested and labeled with the ABO group. The four main phenotypes are A, B, AB, and O, the latter indicating a lack of A and B antigens. The sugars defining A and B antigens are added to carbohydrate chains carrying the H antigen (fucose), which is “hidden” by the A (GalNAc) or B (Gal) sugar. Thus, group A or B erythrocytes appear to have less H antigen than group O cells. Nonetheless, H is found on all human erythrocytes except those from rare individuals of the Oh (Bombay) phenotype.
Anti-A or anti-B immunoglobulins can cause intravascular hemolysis when ABO-incompatible RBCs are transfused. Because A and B antigens also are expressed on most tissue cells, ABO compatibility is a significant consideration in solid-organ transplantation. However, ABO incompatibility only rarely causes severe HDFN because antibodies directed against A and B antigens are predominantly immunoglobulin (Ig) M, which do not cross the placenta, in addition, A and B antigens are not fully developed on RBCs from a fetus (Chap. 55).
Although the ABO blood group system has only four phenotypes, hundreds of alleles have been identified by DNA analyses. The ABO gene was cloned in 1990 following purification of A transferase.17,18 A and B transferases have only four amino acid differences in the catalytic domain, two of which (Leu266Met and Gly268Ala) are primarily responsible for substrate specificity.19 The group O phenotype results from nucleotide changes in A and/or B alleles that cause loss of glycosyltransferase activity. The most common group O (O1) results from a single nucleotide deletion near the 5′ end of the gene that causes a frameshift and early termination with no active enzyme production.20 The ABO gene has seven exons, and A or B subgroups (with only few exceptions) result from a variety of nucleotide changes in exon 7 that cause alterations in the catalytic domain of the glycosyltransferase (reviewed by Chester and Olsson21). The rare B(A), A(B), and cis-AB phenotypes expressing both A and B antigens result from variant glycosyltransferases that have a combination of A- and B-specific residues.21 Numerous common and rare ABO alleles have been reported, and current information is available on websites: and www.isbt-web.org. In addition to nucleotide changes, recombinations and gene rearrangements can result in hybrid alleles that encode for unexpected transferase activity. This situation makes typing of ABO by DNA analysis difficult to interpret.22 The function of the ABO system is not known, although several disease associations are well established.23
The Rh (not Rhesus) system is the second most important blood group system in transfusion medicine because antigen-positive RBCs frequently immunize antigen-negative individuals through transfusion and pregnancy.
Inheritance of Rh antigens is determined by a complex of two closely linked genes: one encodes the protein carrying D antigen (RhD); the other encodes the protein carrying C or c and E or e antigens (RhCE). RBCs from Rh-positive people have both RhD and RhCE, whereas Rh-negative RBCs have only RhCE. In the Rh system, eight common antigen combinations or haplotypes are possible: Dce (R0, Rh0), DCe (R1, Rh1), DcE (R2, Rh2), DCE (RZ, Rhz), ce (r, rh), Ce (r′, rh′), cE (r′′, rh′′), and CE (ry, rhy). The letter “d” is commonly used to designate the lack of D, but there is no d antigen or anti-d.
Several nomenclatures can be used to describe Rh genes and antigens. The Fisher-Race nomenclature, which uses CDE terminology, is more commonly used for antigens; the Wiener nomenclature, which uses Rh/rh (or R/r) designations, is favored for haplotypes and gene complexes; and the Rosenfield and Rubinstein nomenclature, which uses numerical designations, was introduced to allow interpretation without bias.24
The Rh blood group system has over 50 antigens (the ABO system has 4). By far the most important and immunogenic antigen is D (Rh0 in Wiener terminology, referring to Wiener’s discovery that a rhesus monkey injected with human RBCs would produce antibody that agglutinated the RBCs of 85 percent of white New Yorkers). For most clinical purposes, testing individuals for the D antigen and classifying them as D+ (or Rh-positive), or D– (or Rh-negative) is sufficient. Approximately 85 percent of the white population is Rh-positive, and 15 percent is Rh-negative. Most Rh-negative recipients produce anti-D if they receive Rh-positive blood. Anti-D can cause hemolysis in adults following an Rh-mismatched transfusion and in the newborn (HDFN) if antibodies were made by the mother from a prior transfusion or pregnancy. Thus, donors and recipients are routinely typed and matched for D. The risk of anti-D sensitization by transfusion is essentially eliminated by matching. The risk of anti-D sensitization in pregnancy is minimized by passive immunization of mothers at risk against D.
The antigens C, c, E, and e are less immunogenic and become important in patient care only after the corresponding antibody develops or when the basic Rh haplotype must be determined. The remaining 45+ antigens are other Rh protein epitopes whose corresponding antibodies are seldom encountered. Some are encoded by variant Rh alleles and appear as antithetical antigens to C, c, E, or e, or as related “extra” antigens. Others are referred to as compound antigens or cis gene products. For example, the protein produced by RHCE*ce encodes c and e antigens, and the compound f (or ce) antigen. Other compound antigens include Ce (rhi), cE and CE. Still other Rh antigens are related to the complex “mosaic” nature of D and e, and less commonly C, c and E antigens. If immunized, individuals who lack a part of an antigen and who make antibody to the portion they lack, can present with a challenging serologic picture. For example, the D+ person who lacks part of the D antigen and makes an antibody to the missing portion appears to make alloanti-D because normal D+ RBCs carry all D epitopes.25
Some, but not all, individuals who lack part of the D antigen (partial D) have weak expression of D on their red cells that is detected only by the antiglobulin test. Having a RHCE*C gene in trans position to a RHD gene (e.g., Dce/Ce or DCe/Ce genotypes) also can weaken expression of D in some individuals. A third type of weak D expression results from inheriting a RHD gene that encodes all epitopes of D, but in less-than-normal quantity.
DNA analyses have revealed the molecular basis underlying antigens and phenotypes. A list of the alleles that have been described to date is available at: and www.isbt-web.org. Rh blood group orthologs are present in nonhuman primates and other species on the evolutionary tree.26 The RhD and RhCE proteins complex with the Rh-associated glycoprotein (RhAG) in the membrane. RhAG acts as a transporter of gases, most likely for ammonium, nitrous oxide, CO2 and/or O2 but confirmation is needed as to which it is.
In terms of transfusion and HDFN, the other blood group systems and their antigens become clinically relevant only when antibody develops. Transfusion service laboratories identify (antibody identification) the specificity and characterize the reactivity of antibodies detected in routine testing (antibody screening). Once this information is known, the blood bank assesses the clinical significance of the antibody and selects the most appropriate blood for transfusion. Tables 136–1 and 136–2 summarize the number of antigens in each blood group system and other relevant information. A detailed description of all the blood group antigens is beyond the scope of this chapter. Because the molecular bases of most blood group antigens and phenotypes are known,6 DNA analysis can be used to predict the type of transfused patients and to identify the fetus at risk for HDFN.27
GENERAL IMMUNOLOGY OF BLOOD GROUP ANTIGENS
An antigen is a substance that can evoke an immune response when introduced into an immunocompetent host and react with the antibody produced from that immune response. An antigen can have several epitopes, which together are called an antigenic determinant, each of which is capable of eliciting an antibody response.
The ability of an antigen to stimulate an immune response is called immunogenicity, and its ability to react with an antibody is called antigenicity. These primary characteristics are affected by antigen size, shape, rigidity, and the number and location of the determinants on the red cell membrane.
Immunogenicity depends on many antigen characteristics, not just the number of antigen sites. Relative immunogenicity is estimated by comparing the actual observed incidence of an antibody to the calculated likelihood of a possible immunizing event. After A and B, the D antigen is most immunogenic (early work suggested that approximately 80 percent of Rh-negative individuals produce anti-D after receiving a single Rh-positive RBC component but more recent studies indicate it is more like 20 to 30 percent4), followed by K, which stimulates anti-K in approximately 10 percent of cases.16 The antigens c and E are one-third as immunogenic; Fya is one-twenty-fifth as potent; and Jka is one-fiftieth to one-one hundredth times as potent as K.28 It should be noted that immunogenicity does not always correlate with the hemolytic potential of an antibody specificity; for example, K is more immunogenic than Jka but anti-Jka is more likely to cause hemolysis.
ANTIGEN EXPRESSION
The number of antigen sites per RBC has been estimated by measuring the uptake of 125I-labeled antibody or of ferritin-conjugated anti-IgG. Numbers vary widely among blood group systems from a few hundred to over a million (see Table 136–2). Also, estimates for any given antigen can vary greatly.
Most RBC antigens can be detected early in fetal development (A, B, and H antigens can be detected at 5 to 6 weeks’ gestation), but not all are fully developed at birth. A, B, H, I, P1, Lua, Lub, Yta, Xga, Vel, Bg, Doa, Dob, and Kna antigen expression is considerably weaker on cord RBCs than on RBCs from adults. Lea, sometimes Leb, Ch/Rg, AnWj, and Sda, are not readily detectable, although 50 percent of cord samples type Le(a+) with more sensitive test methods. Full expression of A, B, H, I, and Lewis antigens usually is present by age 3 years, whereas full expression of P1 and Lutheran antigens may not occur until age 7 years.
RBCs from individuals who are homozygous for an allele typically have a greater number of antigen sites than do RBCs from individuals who are heterozygous. Consequently, their RBCs can react more strongly with antibody. This difference in expression and antigen–antibody reactivity because of zygosity is known as dosage. For example, RBCs from a homozygous MM individual carry a double dose of M antigen and react more strongly with anti-M than do RBCs from a MN heterozygous individual carrying only a single dose of M. Antithetical antigens C/c, E/e, M/N, S/s, and Jka/Jkb commonly show dosage effect. Dosage is less obvious with D, K/k, and Lua/Lub antigens. It typically is more apparent within a family than between families. Dosage within the Duffy system also may not be serologically obvious because Fy(a+b–) or Fy(a–b+) phenotypes are seen in either homozygous (FyaFya or FybFyb) or hemizygous (FyaFy or FybFy) individuals.
Some blood group antigens are inherited as closely linked genes or haplotypes. Haplotype pairings and gene interaction (either cis or trans) also can affect phenotypic expression. For example, the pairing of RHCE*C in trans position to RHD can result in weak expression of D (see “Rh Blood Group System” above), whereas RHCE*E in cis position with RHD is associated with strong expression of D. Among the common phenotypes, R2R2 RBCs carry the strongest expression of D. In the Kell system, Kpa is associated with weakened expression of in cis k and Jsb antigens.
Still other antigens are affected by regulator genes.29 The dominant type of the Lu(a–b–) phenotype [In(Lu)] results from heterozygosity for an allele of the KLF1 gene, the gene that encodes erythroid Krüppel-like factor (EKLF). The dominant inhibitor gene KLF1 suppresses expression of Lutheran, P1, i, and many other antigens.30 The dominant inhibitor In(Jk) suppresses expression of Jka and Jkb antigens.31 Rare variants of the RHAG gene depress or prevent expression of the Rh antigens (see “Rhnull Syndrome” below).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree