Chapter Outline
Erythrocyte cytochemistry 312
Leucocyte cytochemistry 317
Myeloperoxidase 318
Sudan Black B 319
Neutrophil alkaline phosphatase 319
Acid phosphatase reaction, including tartrate-resistant acid phosphatase reaction 321
Periodic acid–Schiff reaction 321
Esterases 323
Toluidine blue stain 327
Cytochemical reactions and leukaemia classification 327
Acknowledgement
The major contribution of the late Dr David Swirsky to this chapter in previous editions of the book is gratefully acknowledged.
Erythrocyte cytochemistry
Siderocytes and sideroblasts
Siderocytes are red cells containing granules of non-haem iron. They were originally described by Grüneberg in small numbers in the blood of normal rat, mouse and human embryos and in large numbers in mice with a congenital anaemia. The granules are formed of a water-insoluble complex of ferric iron, lipid, protein and carbohydrate. This siderotic material (or haemosiderin) reacts with potassium ferrocyanide to form a blue compound, ferriferrocyanide; this reaction is the basis of a positive Prussian blue (Perls) reaction. The material also stains with Romanowsky dyes and then appears as basophilic granules, which have been referred to as ‘Pappenheimer bodies’ ( Fig. 15-1 ). By contrast, ferritin, which is a water-soluble non-haem compound of iron with the protein apoferritin, is not detectable by Perls reaction. Ferritin is normally present in all cells in the body, whereas in health, haemosiderin is mainly found in macrophages in the bone marrow, liver (Kupffer cells) and spleen. When the body is overloaded with iron, as in haemochromatosis or transfusional haemosiderosis, excess iron is also found in other tissues.
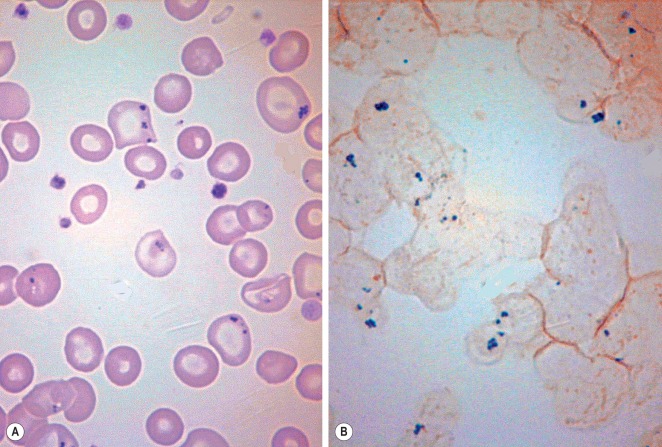
Iron is transported in plasma attached to a β globulin, transferrin, and is taken up selectively by the bone marrow, where the iron–transferrin complex binds to transferrin receptors on the surface of the erythroblast; the iron is released from transferrin and enters the cell. Most of the iron is rapidly converted to haem, synthesis being partly in the cytosol and partly in the mitochondria. The non-haem residue is in the form of ferritin. Degradation of the ferritin turns some of it into haemosiderin, which can be visualised under the light microscope as golden-yellow refractile particles in phagocytic cells. When stained by Perls reaction, haemosiderin is blue.
In health, siderotic granules can normally be seen, in preparations stained by Perls reaction, in the cytoplasm of many of the erythroblasts of human bone marrow and in marrow reticulocytes. However, they are not normally seen in human peripheral blood red cells. After splenectomy, siderocytes can always be found in the peripheral blood, often in large numbers. The reason for this is probably because reticulocytes, after delivery from the marrow, are normally sequestered for a time in the spleen, and there they complete haem synthesis, utilising, for this purpose, the iron stored in their cytoplasm within the siderotic granules. After splenectomy, this stage of reticulocyte maturation has to take place in the bloodstream, with the result that, even in an otherwise healthy person, a small percentage of siderocytes can then be found in the peripheral blood. The spleen is also probably able to remove large siderotic granules – as may be found in disease – from red cells by a process of pitting, and in its absence such granules persist in the red cells throughout their lifespan.
Method for staining siderotic granules
Air dry films of peripheral blood or bone marrow and fix with methanol for 10–20 min. When they are dry, place the slides in a solution of 10 g/l potassium ferrocyanide in 0.1 mol/l HCl made by mixing equal volumes of 47 mmol/l (20 g/l) potassium ferrocyanide and 0.2 mol/l HCl immediately before use.
Leave the slides in the solution for about 10 min at about 20 °C. Wash well in running tap water for 20 min, rinse thoroughly in distilled water and then counterstain with 1 g/l aqueous neutral red or eosin for 10–15 s. Care must be taken to avoid contamination by iron that may have been present on the slides or in staining dishes. Prepare the glassware by soaking in 3 mol/l HCl before washing (see p. 564). For quality control, a positive bone marrow film should always be stained together with the test films.
Prussian blue staining can be applied to films that have previously been stained by Romanowsky dyes, even after years of storage. It is advisable to let the films stand in methanol overnight to remove most of the Romanowsky stain. The film should be checked before carrying out Perls reaction to ensure that there is no residual blue staining that could obscure Prussian blue staining. Sundberg and Bromann described a technique whereby films were stained first by a Romanowsky dye (Wright stain) and then overstained by the acid–ferrocyanide method. This can give beautiful pictures, but the small blue-stained iron-containing granules tend to be masked in young erythroblasts by the general basophilia of the cell cytoplasm. Hayhoe and Quaglino described a method for combined periodic acid–Schiff (PAS) and iron staining. This may be helpful in the investigation of abnormal erythropoiesis in which the erythroblasts give a positive PAS reaction (see p. 321). A rapid method has been described for demonstrating siderotic granules by staining with 1% bromochlorphenol blue for 1 min. Iron-containing granules stain dark purple.
Significance of siderocytes
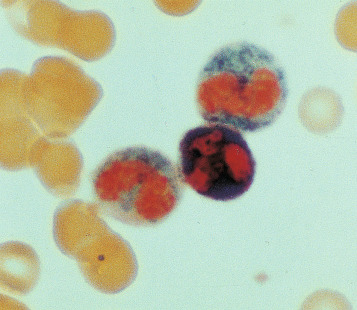
Siderocytes contain one or two (rarely many) small, unevenly distributed iron- containing granules that stain a Prussian blue colour. There are normally a few very small scattered siderotic granules in about 40% of late erythroblasts. They stain faintly and may be difficult to see by light microscopy. The percentage of erythroblasts recognisable as sideroblasts is increased in haemolytic and megaloblastic anaemias and in haemochromatosis and haemosiderosis, in proportion to the degree of saturation of transferrin (i.e. to the amount of iron available). A disproportionate increase in the percentage of erythroblasts that are sideroblasts occurs when the synthesis of haemoglobin is impaired, in which case the siderotic granules are both more numerous and larger than normal ( Fig. 15-2 ). When there is a defect in haem synthesis, the granules are deposited in mitochondria and frequently appear to be arranged in a collar around the nucleus ( Fig. 15-3 ), giving the ‘ring sideroblasts’ characteristic of sideroblastic anaemias. In contrast, the distribution of the granules within the cell tends to be mainly normal in conditions in which globin synthesis alone is affected (e.g. in thalassaemia) or when there is iron overload.
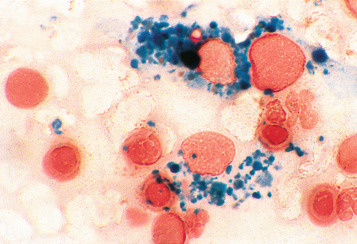
There are several types of sideroblastic anaemia. These include the congenital (hereditary) type, pyridoxine (vitamin B 6 ) deficiency (rarely), sideroblastic anaemia caused by B 6 antagonists (e.g. drugs used in antituberculosis therapy) and secondary sideroblastic anaemia in alcoholism and lead poisoning. The presence of ring sideroblasts is a defining feature of refractory anaemia with ring sideroblasts and refractory cytopenia with multilineage dysplasia and ring sideroblasts, two of the World Health Organisation (WHO) categories of myelodysplastic syndrome (MDS). They may also occur in other categories of MDS. Ring sideroblasts are not uncommon in other haematological neoplasms, including primary myelofibrosis and acute myeloid leukaemia (AML), particularly erythroleukaemia and the WHO categories of therapy-related AML and AML with multilineage myelodysplasia. Ring sideroblasts have been defined as erythroblasts with at least five siderotic granules surrounding at least one-third of the nucleus. ,
In sideroblastic anaemia as a feature of a haematological neoplasm, erythroblasts at all stages of maturity may be loaded with siderotic granules, whereas in the secondary sideroblastic anaemias and in the hereditary types, the more mature cells seem most affected.
In addition to the siderotic granules within erythroblasts, haemosiderin can normally be seen in marrow films as accumulations of small granules, lying free or in macrophages in marrow fragments. The amount of haemosiderin will be markedly increased in patients with increased iron stores, whereas haemosiderin is absent in iron deficiency anaemia ( Fig. 15-4 ). In practice, staining to demonstrate iron stores in marrow fragments and siderotic granules in erythroblasts is a simple and valuable diagnostic procedure and should be applied as a routine to marrow films from the initial bone marrow aspirate of each patient.
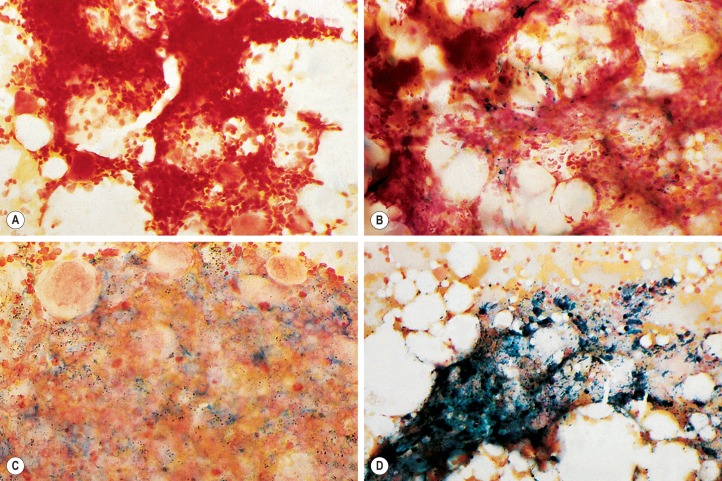
In chronic infections and in other examples of anaemia of chronic disease, the iron stores may be increased, with much siderotic material in macrophages but little or none visible in erythroblasts. Markedly excessive iron in macrophages is also a feature of thalassaemia intermedia, thalassaemia major and some dyserythropoietic anaemias. Conversely, absence of iron is diagnostic of iron deficiency or iron depletion (the latter term indicating the state in which storage iron is absent but anaemia is not yet evident). One study has shown that to establish the absence of stainable iron, at least seven particles must be examined, if necessary using more than one slide for this purpose.
Occasionally a Perls stain demonstrates haemosiderin in plasma cells (e.g. in copper deficiency, alcoholism and conditions of iron overload).
There is no cytochemical method for demonstrating ferritin.
Haemoglobin derivatives
Heinz bodies in red cells
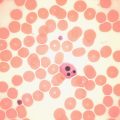
Heinz, in 1890, was the first to describe in detail inclusions in red cells developing as the result of the action of acetylphenylhydrazine on the blood. It is now known that Heinz bodies can be produced by the action on red cells of a wide range of aromatic nitro- and amino-compounds, as well as by inorganic oxidising agents such as potassium chlorate. They also occur when one or other of the globin chains of haemoglobin is unstable. In man, the finding of Heinz bodies is a sign of either chemical poisoning, drug toxicity, glucose-6-phosphate dehydrogenase (G6PD) deficiency or the presence of an unstable haemoglobin (e.g. haemoglobin Köln). When of chemical or drug origin, Heinz bodies are likely to be visible in red cells only if the patient has been splenectomised previously or when large doses of the chemical or drug have been taken. When they are due to an unstable haemoglobin, they are rarely visible in freshly withdrawn red cells except after splenectomy. They may nevertheless develop in vitro in the blood of patients who have not been splenectomised if the specimen is incubated for 24–48 h. Heinz bodies are a late sign of oxidative damage and represent an end-product of the degradation of haemoglobin. In acute oxidant damage, the presence of Heinz bodies can be suspected from a Romanowsky-stained blood film, when they appear as small inclusions protruding from irregularly contracted cells or within an otherwise empty cell membrane. Their nature can be confirmed as discussed below. Reviews dealing with Heinz bodies include those by Jacob and by White.
Demonstration of Heinz bodies
Unstained preparations
Heinz bodies may be seen as refractile objects in dry, unstained films, if the illumination is reduced by lowering the microscope condenser. They also can be seen by dark-ground illumination or phase-contrast microscopy. However, it is preferable to look for them in stained preparations (see below). In size they vary from 1 to 3 μm. One or more may be present in a single cell. They are usually close to the cell membrane and may cause a protrusion of the membrane; in wet preparations, they may move around within the cells in a slow Brownian movement.
The degradation product of an unstable haemoglobin (e.g. haemoglobin Köln) exhibits green fluorescence when excited by blue light at 370 nm in a fluorescence microscope.
Stained preparations
Dissolve approximately 0.5 g of methyl violet in 100 ml of 9 g/l NaCl and filter. Add 1 volume of blood (in any anticoagulant) to 4 volumes of the methyl violet solution and allow the suspension to stand for about 10 min at room temperature. Then prepare films and allow them to dry or view the suspension of cells between slide and coverslip. The Heinz bodies stain an intense purple ( Fig. 15-5 ).
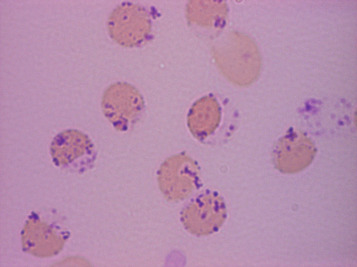
Heinz bodies also stain with other basic dyes. Brilliant green stains them well, and none of the stain is taken up by the remainder of the red cell. Rhodanile blue (5 g/l solution in 10 g/l NaCl) stains them rapidly (i.e. within 2 min), at which time reticulocytes are only weakly stained. Compared with methyl violet, Heinz bodies stain less intensely with brilliant cresyl blue or New methylene blue. Nevertheless, they may be readily seen as pale blue bodies in a well-stained reticulocyte preparation, if the preparation is not counterstained.
If permanent preparations are required, fix the vitally stained films by exposure to formalin vapour for 5–10 min. Then counterstain the fixed films with 1 g/l eosin or neutral red, after thoroughly washing in water. If films are fixed in methanol, Heinz bodies are decolourised.
In β thalassaemia major, methyl violet staining of the bone marrow will demonstrate precipitated α chains. These appear as large irregular inclusions in late normoblasts, usually single and closely adhering to the nucleus. If such patients are splenectomised, inclusions are also found in reticulocytes and mature red blood cells, in the circulation as well as in the bone marrow.
Carboxyhaemoglobin and methaemoglobin
Carboxyhaemoglobin- and methaemoglobin-containing cells can be demonstrated cytochemically. These methods are described by Kleihauer and Betke. They have little practical value in modern practice.
Fetal haemoglobin
An acid-elution cytochemical method that was introduced by Kleihauer et al . is a sensitive procedure to identify individual cells containing haemoglobin F even when few are present. Their detection in the maternal circulation has provided valuable information on the pathogenesis of haemolytic disease of the newborn.
The identification of cells containing haemoglobin F depends on the fact that they resist acid elution to a greater extent than normal cells; thus, in the technique described below, they appear as isolated, darkly stained cells among a background of palely staining ghost cells. The occasional cells that stain to an intermediate degree are less easy to evaluate; some may be reticulocytes because these also resist acid elution to some extent. The following method, in which elution is carried out at pH 1.5, is recommended.
Reagents
- •
Fixative. 80% ethanol
- •
Elution solution. Solution A: 7.5 g/l haematoxylin in 90% ethanol. Solution B: FeCl 3 , 24 g; 2.5 mol/l HCl, 20 ml; doubly distilled water to 1 litre.
For use, mix well 5 volumes of A and 1 volume of B. The pH is approximately 1.5. The solution can be used for about 4 weeks; if a precipitate forms, the solution should be filtered.
Counterstain. 1 g/l aqueous erythrosin or 2.5 g/l aqueous eosin.
Method
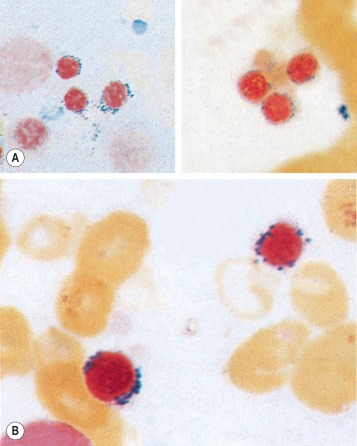
Prepare fresh air-dried films. Immediately after drying, fix the films for 5 min in 80% ethanol in a Coplin jar. Rinse the slides rapidly in water and stand them vertically on blotting paper for about 10 min to dry. Place the slides for 20 s in a Coplin jar containing the elution solution. Wash the slides thoroughly in water and finally place them in the counterstain for 2 min. Rinse in tap water and allow them to dry in the air. Films prepared (1) from a mixture of cord blood and adult blood and (2) from normal adult blood should be stained alongside the test films as positive and negative controls, respectively. In fetomaternal haemorrhage, the maternal blood shows two populations of cells; fetal cells stain red and adult ghost cells stain pale pink ( Fig. 15-6 , A ). This appearance must be distinguished from that of hereditary persistence of fetal haemoglobin, when distribution may be heterogeneous ( Fig. 15-6 , B ) or all cells may be uniformly red (pancellular distribution).
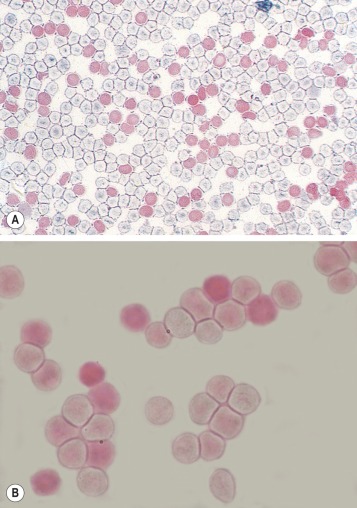
A number of modifications of the Kleihauer method have been proposed. In one, New methylene blue is incorporated in the buffer solution, the reaction time is prolonged and buffer is used for washing the films. The advantage of this technique is that reticulocytes stain blue, whereas cells containing haemoglobin F stain pink.
An immunofluorescent staining method has been developed based on the use of a specific antibody against haemoglobin F, which does not react with haemoglobin A. By using a double-labelling procedure with rhodamine-labelled antibody against γ globin and a fluorescein-labelled antibody against β globin, it is possible to detect the presence of haemoglobin F and haemoglobin A in the same cell.
Haemoglobin S and other haemoglobin variants
Immunodiffusion with specific antibodies has been used for the identification of haemoglobin S, haemoglobin A 2 and haemoglobin F in red cells. , An alternative method is by detection of cells after labelling the cells with fluorescein isothiocyanate (FITC). By a double-labelling method similar to that described earlier, it is possible to identify haemoglobin S as well as another haemoglobin in individual cells.
Leucocyte cytochemistry
Leucocyte cytochemistry encompasses the techniques used to identify diagnostically useful enzymes or other substances in the cytoplasm of haemopoietic cells. These techniques are particularly useful for the characterisation of immature cells in AML and the identification of maturation abnormalities in MDS and myeloproliferative neoplasms (MPN). There are many variations in the staining techniques, as discussed in the recommendations of an Expert Panel of the International Committee (now Council) for Standardisation in Haematology. , Detailed reference works discussing the theoretical and practical aspects of cytochemistry are available. The use of cytochemistry to characterise lymphoproliferative disorders has been largely superseded by immunological techniques (see Chapter 16 ). The results of cytochemical tests should always be interpreted in relation to Romanowsky stains and immunological techniques. Control blood or marrow slides should always be stained in parallel to ensure the quality of the staining. The principal uses of cytochemistry are as follows:
- 1.
To characterise the blast cells in acute leukaemia as myeloid (leading to a diagnosis of AML unless there is also evidence of lymphoid differentiation)
- 2.
To demonstrate myeloperoxidase or nonspecific esterase activity and thus contribute to a diagnosis of mixed-phenotype acute leukaemia, according to the criteria of the 2008 WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues
- 3.
To identify granulocytic and monocytic components in AML
- 4.
To identify unusual lineages occasionally involved in clonal myeloid disorders (e.g. basophils and mast cells)
- 5.
To detect cytoplasmic abnormalities and enzyme deficiencies in myeloid disorders (e.g. myeloperoxidase- deficient neutrophils in MDS or acute leukaemia, neutrophil alkaline phosphatase-deficient neutrophils in chronic myelogenous leukaemia, CML)
- 6.
To identify Auer rods in MDS (and thus classify a case as refractory anaemia with excess of blasts II in the WHO classification)
- 7.
To confirm a diagnosis of hairy cell leukaemia.
It is particularly important that cytochemistry is not neglected in under-resourced countries when immunophenotyping is not readily available.
Myeloperoxidase
Myeloperoxidase (MPO) is located in the primary and secondary granules of neutrophils and their precursors, in eosinophil granules and in the azurophilic granules of monocytes. The MPO in eosinophil granules is cyanide resistant, whereas that in neutrophils and monocytes is cyanide sensitive. MPO splits H 2 O 2 and in the presence of a chromogenic electron donor forms an insoluble reaction product. Various benzidine substitutes have been used, of which 3,3′-diaminobenzidine (DAB) is the preferred chromogen. , The reaction product is stable, insoluble and nondiffusible. Staining can be enhanced by immersing the slides in copper sulphate or nitrate, but this is generally not required in normal diagnostic practice. Alternative non-benzidine-based techniques use 4-chloro-1-naphthol (4CN) or 3-amino- 9-ethylcarbazole. The former gives very crisp staining but is soluble in some mounting media and immersion oil; the latter shows some diffusibility and does not stain as strongly as DAB.
Method with 3,3′-diaminobenzidine
Reagents
- •
Fixative. Buffered formal acetone (BFA) (see p. 564)
- •
Substrate. 3,3′-DAB (Sigma D-8001, Sigma-Aldrich, UK)
- •
Buffer. Sorensen phosphate buffer, pH 7.3 (see p. 563)
- •
Hydrogen peroxide (H 2 O 2 , 30% w/v)
- •
Counterstain. Aqueous haematoxylin
Method
- 1.
Fix air-dried smears for 30 s in cold BFA.
- 2.
Rinse thoroughly in gently running tap water and air dry.
- 3.
Incubate for 10 min in working substrate solution. Thoroughly mix 30 mg DAB in 60 ml buffer, add 120 μl H 2 O 2 and mix well.
- 4.
Counterstain with haematoxylin for 1–5 min, rinse in running tap water and air dry.
Technical considerations.
MPO is not inhibited by heparin, oxalate or EDTA anticoagulants. Films should be made within 12 h of blood collection. Staining is satisfactory on films kept at room temperature for at least a week. The DAB should be stored frozen at − 20 °C in 1 ml aliquots of 30 mg in 1 ml of buffer. For optimum results, it is essential to dissolve the DAB thoroughly in the buffer and to ensure the reagents in the incubation mixture are well mixed. The stain is robust and not strictly pH dependent, with identical results being obtained when using buffers ranging in pH from 7.0 to 9.0. The counterstaining time should be adjusted to the minimum time to give clear nuclear detail. Methyl green is an alternative counterstain, giving excellent contrast with the DAB reaction product, but nuclear detail is more difficult to discern.
Results and interpretation.
The reaction product is brown and granular ( Fig. 15-7 , A ). Red cells and erythroid precursors show diffuse brown cytoplasmic staining. The most primitive myeloblasts are negative, with granular positivity appearing progressively as they mature toward the promyelocyte stage. The positivity may be localised to the Golgi region. Promyelocytes and myelocytes are the most strongly staining cells in the granulocyte series, with positive (primary) granules packing the cytoplasm. Metamyelocytes and neutrophils have progressively fewer positive (secondary) granules. Eosinophil granules stain strongly and the large specific eosinophil granules are easily distinguished from neutrophil granules. Eosinophil granule peroxidase is distinct biochemically and immunologically from neutrophil peroxidase. Monoblasts and monocytes may be negative or positive. When positive, the granules are smaller than in neutrophils and diffusely scattered throughout the cytoplasm. MPO activity is present in basophil granules but is not demonstrable in mature basophils by the DAB reaction described earlier.
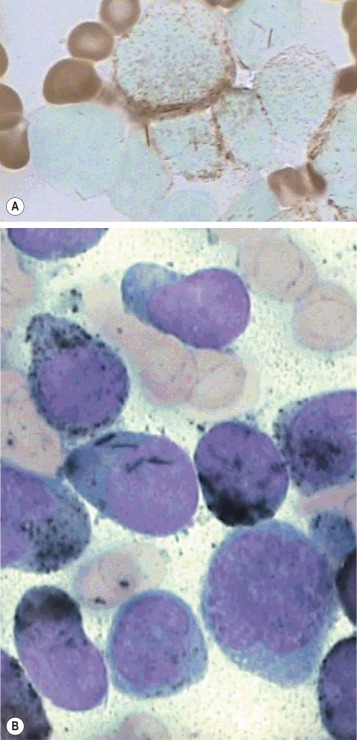
Pathological variations.
Some individuals have congenital deficiency of neutrophil MPO. All stages of the neutrophil lineage, from the myeloblast onward, are negative. In these individuals, the eosinophils stain normally. Other individuals have an MPO deficiency confined to eosinophils or monocytes. Dysplastic neutrophils may be MPO negative. Auer rods stain well with DAB and are seen more frequently on MPO staining than on Romanowsky-stained films.
Sudan Black B
Sudan Black B (SBB) is a lipophilic dye that binds irreversibly to an undefined granule component in granulocytes, eosinophils and some monocytes. It cannot be extracted from the stained granules by organic dye solvents and gives comparable information to that of MPO staining. The currently used staining solution is essentially that described by Sheehan and Storey.
Reagents
- •
Fixative. Vapour from 40% formaldehyde solution
- •
Stain. SBB (Sigma S-2380) 0.3 g in 100 ml absolute ethanol
- •
Phenol buffer. Dissolve 16 g crystalline phenol in 30 ml absolute ethanol. Add to 100 ml distilled water in which 0.3 g Na 2 HPO 4 .12H 2 O has been dissolved
- •
Working stain solution. Add 40 ml buffer to 60 ml SBB solution
- •
Counterstain. May–Grünwald–Giemsa or Leishman stain (see p. 52)
Method
- 1.
Fix air-dried smears in formalin vapour as follows. Place a small square of filter paper in the bottom of a Coplin jar. Add 2 drops of 40% formalin, put on the lid and leave for 15 min to allow vaporisation. Place the slides in the Coplin jar and replace the lid. After 5–10 min, remove the slides and stand on end for 15 min to ‘air wash’.
- 2.
Immerse the slides in the working stain solution for 1 h in a Coplin jar with a lid on.
- 3.
Transfer slides to a staining rack and immediately flood with 70% alcohol. After 30 s, tip the 70% alcohol off and flood again for 30 s. Repeat three times in total.
- 4.
Rinse in gently running tap water and air dry.
- 5.
Counterstain without further fixation with Leishman stain or May–Grünwald–Giemsa.
Technical considerations
Buffered formal acetone fixation for 30 s is a satisfactory alternative to formalin vapour. The working stain solution should be replaced after 4 weeks. Bone marrow films with fatty particles containing lipid-soluble SBB benefit from a 5 s swirl in xylene followed by rinsing in running tap water and air drying prior to counterstaining. The Romanowsky counterstain gives excellent cytological detail of all cells present.
Results and interpretation
The reaction product is black and granular. The results are essentially similar to those seen with MPO staining, both in normal and leukaemic cells ( Fig. 15-7 , B ). MPO-negative neutrophils are also SBB negative. The only notable difference is in eosinophil granules, which have a clear core when stained with SBB. Rare cases (1% to 2%) of acute lymphoblastic leukaemia (ALL) show nongranular smudgy positivity not seen with MPO staining. Basophils are generally not positive but may show bright red/purple metachromatic staining of the granules.
Neutrophil alkaline phosphatase
Alkaline phosphatase activity is found predominantly in mature neutrophils, with some activity in metamyelocytes. Although demonstrated as a granular reaction product in the cytoplasm, enzyme activity is associated with a poorly characterised intracytoplasmic membranous component distinct from primary or secondary granules. Other leucocytes are generally negative, but rare cases of lymphoid malignancies show cytochemically demonstrable activity. Bone marrow macrophages are positive. Early methods of demonstrating alkaline phosphatase relied on the use of glycerophosphate or other phosphomonoesters as the substrate at alkaline pH, with a final black reaction product of lead sulphide. Azo-dye techniques are simpler, giving equally good results. These methods use substituted naphthols as the substrate and it is the liberated naphthol rather than phosphate that is used to combine with the azo-dye to give the final reaction product.
Reagents
- •
Fixative. 4% formalin methanol. Add 10 ml 40% formalin to 90 ml methanol. Keep in a freezer. Discard after 2 weeks.
- •
Substrate. Naphthol AS phosphate (Sigma N-5625). Store in freezer.
- •
Buffer. 0.2 mol/l Tris buffer, pH 9.0 (see p. 564)
- •
Stock substrate solution. Dissolve 30 mg naphthol AS phosphate in 0.5 ml N,N-dimethylformamide (Sigma D-4551). Add 100 ml 0.2 mol/l Tris buffer, pH 9.1. Store in a refrigerator at 2–4 °C. The solution is stable for several months.
- •
Coupling azo-dye. Fast Blue BB salt (Sigma F-0250). Store in freezer.
- •
Counterstain. Neutral red, 0.02% aqueous solution
Method
- 1.
Fix freshly made air-dried blood films for 30 s in cold 4% formalin methanol.
- 2.
Rinse with tap water and air dry.
- 3.
Prepare working substrate solution by allowing 40 ml of stock substrate solution to warm to room temperature. Add 24 mg of Fast Blue BB and mix thoroughly until dissolved. Incubate slides for 15 min.
- 4.
Wash in tap water and air dry.
- 5.
Counterstain for 3 min in 0.02% aqueous neutral red, rinse briefly and air dry.
Technical considerations
N,N-dimethylformamide may dissolve some types of plastic; therefore a glass tube should be used to dissolve the substrate. Blood films should be made soon after blood collection, preferably within 30 min because neutrophil alkaline phosphatase (NAP) activity decreases rapidly in EDTA-anticoagulated blood. Once spread, the blood film should be stained within 6 h. A control film with a predictably high score (e.g. from a patient with reactive neutrophilia or from a pregnant woman) should be processed together with the patient film. The technical aspects of blood film preparation and the effects of fixation on NAP activity are discussed by Kaplow.
Results and interpretation
The reaction product is blue and granular. The intensity of reaction product in neutrophils varies from negative to strongly positive, with coarse granules filling the cytoplasm and overlying the nucleus ( Fig. 15-8 ). An overall score is obtained by assessing the stain intensity in 100 consecutive neutrophils, with each neutrophil scored on a scale of 0–4 as follows:
0 – Negative, no granules
1 – Occasional granules scattered in the cytoplasm
2 – Moderate numbers of granules
3 – Numerous granules
4 – Heavy positivity with numerous coarse granules crowding the cytoplasm, frequently overlying the nucleus.