- Type 1 diabetes mellitus (T1DM) develops in genetically susceptible individuals after a preclinical phase of variable length usually with immune-mediated destruction of pancreatic β-cells, and requires lifelong treatment with insulin.
- The disease can occur at any age, but the incidence peaks around puberty. Classification of T1DM vs type 2 diabetes (T2DM) becomes increasingly difficult with age.
- In childhood, the incidence is similar in females and males, but there is a 1.3- to 2.0-fold male excess in incidence after about 15 years of age in most populations.
- The incidence in childhood varies enormously between countries. Some Asian and South American populations have low incidences (approximately 0.1–8 per 100 000/year), while Finland (>40 per 100 000/year), Sardinia (approximately 38 per 100 000/year) and Sweden (approximately 30 per 100 000/year) have high incidences. North America (15–25 per 100 000/year) and Australia (approximately 15 per 100 000/ year) have moderate to high incidences, while eastern countries in Europe have low to moderate incidences (4–10 per 100 000/year).
- About 10–20% of newly diagnosed childhood cases of T1DM have an affected first-degree relative. Those with an affected sibling or parent have a cumulative risk of 3–7% up to about 20 years of age, compared to cumulative risks of 0.2–0.8% in the respective general populations. The cumulative incidence among the monozygotic co-twins of persons with T1DM is less than 50%, even after >30 years’ follow-up.
- Some of the geographic differences and familial aggregation may be explained by human leukocyte antigen haplotypes.
- The incidence of childhood-onset T1DM has increased 3–4% per calendar year, and there is a tendency towards younger average age at onset over time which cannot be explained by genetic factors. The causes of this increasing trend are not known.
- Virus infections and nutritional factors have been implicated, but no specific environmental factor has been established as a risk factor.
- Even though insulin replacement therapy and other advances in the management of T1DM have improved the prognosis of patients with T1DM, their mortality is still at least two times (approximately 2- to 10-fold) higher than in the background population. This is because of both acute and chronic complications of the disease, including cardiovascular disease after about 30 years of age.
Introduction
Type 1 diabetes mellitus (T1DM) requires lifelong treatment with insulin, and in the majority of cases results from a cell-mediated autoimmune destruction of the β-cells in susceptible individuals. This occurs after a long but variable preclinical period when islet autoantibodies to insulin, glutamine acid decarboxylase (GAD) and insulinoma-associated antigen 2 (IA-2) and other autoantigens can be detected [1]. Autoantibodies are not thought to cause the disease, but are markers of ongoing β-cell destruction. In general, the distinction between autoimmune T1DM (type 1 A according to the WHO [2]) and non-autoimmune T1DM (type 1 B) is not necessarily useful for clinicians or epidemiologists. Problems with measurement error of autoantibodies, the likely existence of additional autoantibodies (as indicated by the recent discovery of zinc transporter 8 autoantibodies [3]) and, perhaps equally important, the transient nature of autoantibodies, make accurate estimates of the proportion of autoimmune T1DM problematic. From longitudinal studies such as DAISY, it is known that some patients with newly diagnosed T1DM are negative for all autoantibodies at diagnosis while having previously been positive.
Genetic factors influence the susceptibility to T1DM, particularly human leukocyte antigen (HLA) genes [4,5]. It is particularly the combination of HLA DR3-DQ2 and DR4-DQ8 that confers very high risk of T1DM, while those who carry only one of the two risk haplotypes have moderately increased risk. More details on the role of genetic factors and the pathologic process are covered in the first part of Chapter 9. Diagnosis of T1DM among children is considered relatively simple, typically with very high glucose levels and clear dependence upon insulin, but classification and early detection becomes increasingly difficult with increasing age.
Occurrence of T1DM by age, sex, place and time
The World Health Organization (WHO) DIAMOND Study (Multinational Project for Childhood Diabetes) [6] and the EURODIAB ACE Study [7,8] have collected standardized incidence data for T1DM among children aged under 15 years, based on notification by the diagnosing physician and date of diagnosis, defined as the date of first insulin injection. In both projects, the degree of undercounting of cases has been estimated using a second source of information [9].
Incidence rates are calculated as the number of new cases per 100 000 person -years, where person-years are estimated as the number of individuals in the population contributing the incident patients during the study period (mean population size in each calendar year, sex and age group). The proportion of the population expected to develop the disease by a certain age can be approximated by multiplying the average incidence rate in an age group by the number of years covered by the age group, the cumulative incidence rate. For instance, if the average incidence rate among 0-to 14-year-olds is 20 per 100 000 person-years, then ([20/100 000] x 15 = 0.003 =) 0.3% of children in that popul ation will develop disease before they reach 15 years of age. The prevalence of disease among 0-to 14-year-olds is thus less than the cumulative incidence up to 15 years of age.
Most of the incidence data available today come from studies of children in European countries. Incidence data from Africa are still sparse, but increased information on the incidence of T1DM among Asian and South American populations has changed the understanding of the global patterns in variation in incidence.
Occurrence of T1DM by age
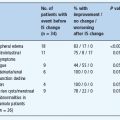
In principle, T1DM may occur at any age, but it is very rare in the first year of life and becomes increasingly difficult to distinguish from other types of diabetes after about 30 years of age. Essentially all populations display a steady increase in incidence rate with age up to around 10–15 years [6], but recent data from Finland indicate an incidence in 0- to 4-year-olds that is nearly as high as that in 10- to 14-year-olds [10]. The incidence rate increases from birth to peak at around puberty (Figure 3.1) and in most populations is lower among 15 – to 29 -year- olds than among 0-to 14-year-olds [11–15]. In some populations there seems to be a second rise in incidence after the age of about 25–30 years [16–18].
In the Swedish nationwide prospective incidence study of 15-to 34-year-olds, 78% of the newly diagnosed patients were classified as having T1DM and 15% as having type 2 diabetes mellitus (T2DM) at the time of the diagnosis [19]. The follow-up showed that 92% of patients diagnosed before 30 years of age were treated with insulin at a later date [20]. These findings are consistent with recent incidence data from Finland among 15- to 39-year-olds [21,22]. In these studies, the incidence of T2DM exceeded that of T1DM by the age of about 30 years. By contrast, in Turin, Italy, which has a much lower incidence of T1DM, the incidence of T2DM is about three times higher than that of T1DM at the age of 30 years [16]. Although beyond the scope of this chapter, it is important to consider the possible clinical heterogeneity and increasing difficulty of classification of diabetes with increasing age [23,24]. A proportion of the apparent heterogeneity may represent extremes within a continuum, although there is a need for more data in this field [24].
Figure 3.1 Incidence rate of type 1 diabetes per 100 000/year in Swedish males and females by age during 1983–1998. Reproduced from Pundziute-Lyckå et al. [14], with permission from Springer-Verlag.
There is a lack of population-based incidence data for age groups above 35 years of age. The only published population-based incidence studies covering the incidence of T1DM over the whole age span is one from Rochester, Minnesota, 1945–1969 [25] and from Denmark, 1973–1977 [26]. The latter study indicated that the cumulative probability of developing T1DM before age 80 years is in the range of 1–1.5%.
Incidence by sex
The peak in incidence rate among children occurs slightly earlier in girls than boys (Figure 3.1), suggesting an influence of puberty. During the 1970s there was a male excess of T1DM in children in populations of European origin and a female excess in populations of African and Asian origin [27]; however, during the early 1990s, the sex-specific pattern in incidence among children changed towards more modest differences. Of 112 centers with data during 1990–1999, only six showed a significantly excess of females (Beijing, Hong Kong and Zunyi in China, New South Wales in Australia, Puerto Rico and the Afro-American ethnic group in the USA) and six centers showed a significant male excess (West Bulgaria, Finland, Attica in Greece, Switzerland, Oxford in the UK and the Dominican Republic), and all differences were generally of modest magnitude [6]. The general impression is still that it is the high incidence countries that tend to have a slight male excess, while the opposite is seen in low incidence countries. Many studies have shown a male excess in the incidence of T1DM among young adults [15,28]. While the male: female ratio among children in most populations is 0.9–1.1 [6], the male: female ratio among young adults ranges from about 1.3 up to 2.0 in many populations [15,28].
Incidence by country
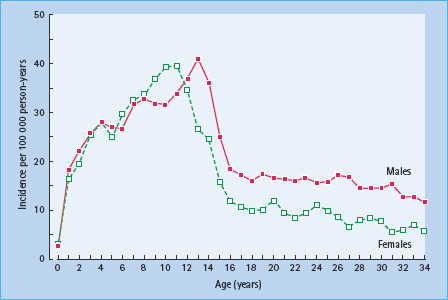
The incidence rate of T1DM among childhood populations shows a vast geographic variation worldwide (Figure 3.2) [68,29]. Over the period 1990–1999, the age-adjusted incidence rate of T1DM ranged globally from 0.1 in Zunyi (China) and Caracas (Venezuela) to 37.8 in Sardinia and 40.9 per 100 000/year in Finland [6]. The most recent data from Finland indicate an incidence rate of nearly 60 per 100 000 in 2006 [10]. the highest incidence ever recorded.
Figure 3.2 Geographic variation in childhood onset type 1 diabetes incidence rates, 1990–1999. Note that most registries were not nationwide and that several countries display within-country variation. Reproduced from the Diamond Project Group [6] with permission from John Wiley & Sons Ltd, Chichester.
European countries are well represented with registries, and there is an approximately 10-fold difference between highest and lowest incidence countries in Europe [7], with the apparently lowest rate of 3.6 per 100 000/year in Macedonia during 19891994. Sweden has a high incidence rate (30 per 100 000/years). Centers from France and mainland Italy, for instance, report intermediate incidence rates around 10 per 100 000/year, while some other European countries have intermediate to high incidence rates [6]. In general, the incidence rates are low in eastern European countries, although the most recent EURODIAB data show that for several countries where the incidence rate previously was below 10 per 100 000/year, the incidence rate during 1999–2003 has increased to 10 per 100 000/year or more, for example in Lithuania, Bucharest in Romania and in Katowice in Poland [8].
Standardized data for the age group 15–29 years from European centers (in Belgium, Lithuania, Romania, Sardinia, Slovakia, Spain, Sweden and the UK) during 1996–1997 showed incidence rates between 5 and 12 per 100 000/year [15]. Incidence rates among 15- to 29-year-olds within this range have also been reported, albeit from earlier time periods, in other European centers, such as 5.5 per 100 000/year in Rzeszow, Poland, 1980–1992 [30], appproximately 7 per 100 000/year in Turin, mainland Italy 1984–1991 [16,31] and approximately 13 per 100 000/year in two regions of Denmark, 1970–1976 [11].
While Sweden and Sardinia have higher incidence rates among children, the incidence rate among young adults was not much higher in Sardinia and Sweden than the other centers in the multicenter study [15]. Older data from Norway (1978–1982) [13] indicated a higher incidence rate of 17 per 100 000/year and recent data from Finland (1992–2001) [21] indicated an incidence rate of 18 per 100 000/year among 15- to 29-year-olds. Differences between countries in the incidence among young adults should be interpreted with caution until more data are collected using comparable methodology.
The USA is represented with the data collected during the 1990s from Allegheny County in Pennsylvania, Chicago and Jefferson County in Alabama, with incidence rates in the range 11–18 per 100 000/year among children. Alberta and Calgary in Canada had slightly higher rates (about 23 per 100 000/year; Figure 3.2) [6], while the data from the Avalon Peninsula, Newfoundland, indicated a higher incidence rate of 35.9 per 100 000/year [32].
In South America, children in centers in Venezuela, Paraguay and Colombia had incidence rates of less than 1 per 100 000/year, Chile (Santiago) had around 4 per 100 000/year, while Argentina and Brazil had an average of around 8 per 100 000/year. Many centers in Central America and the West Indies (except Puerto Rico and St. Thomas) generally have low to intermediate incidence rates, with indications of decreasing time trends [6].
Data from New Zealand and parts of Australia show moderate to high incidence rates (15–25 per 100 000/year) among children. 6]. Data from other Pacific Island countries are lacking, and would be difficult to interpret because the populations in most island countries are small. In Asia, the mean incidence rate among the 23 centers in China during the early 1990s was 0.8 per 100 000/years. Japan has a slightly higher average incidence rate of 1.7 per 100 000/year among three centers, while Kuwait stands out with a high incidence rate of 22 per 100 000/year.
There is limited information on the incidence rate of T1DM from sub-Saharan Africa [33]. The five DIAMOND centers are all in North Africa (Algeria, Libya, Sudan and Tunisia) or Mauritius, an island off the coast of Madagascar. Incidence rates reported from these centers range from low to intermediate, but these countries cannot be said to be representative of Africa.
Variation in incidence within countries, including by ethnic group
In several countries, marked within-country variations in the incidence rate of T1DM have been reported. For instance, up to 1.5-fold differences have been described within relatively homogenous populations, such as Finland, Sweden and Norway [34–36]. In Italy, the incidence rate among children in Sardinia 1990–1999 (38 per 100 000/year) was three to six times higher than the average in mainland Italy, where the incidence rates vary twofold from 6.3 in Campania to 12.2 per 100 000/year in Pavia in the same period [37]. In China, there was a 12-fold geographic variation (0.13–1.61 per 100 000), generally with higher incidence in the north and the east. In addition, there was a sixfold difference between the Mongol (1.82 per 100 000) and the Zhuang (0.32 per 100 000/year) ethnic groups [38].
The variation in incidence within some countries may in part be because of ethnic heterogeneity of populations. For instance, the 1.5-fold higher incidence among children in the South Island of New Zealand compared with that in the North Island was largely explained by the 4.5 times higher incidence among children of European origin compared to that among Maoris [39].
Several methodological problems arise when making inferences based on epidemiologic studies of different ethnic groups and immigrants. These include differential ascertainment and definition of ethnic group, underestimation of the base population in some ethnic groups, genetic admixture and possible heterogeneity in clinical presentation [24,40,41]. Among childhood onset cases, however, it seems that the large majority, even in Japan [42], have classic autoimmune T1DM. Historically, the incidence of T1DM has been higher in populations of European origin, particularly those living in Europe, than that among populations of non-European origin [40,41]. Despite the previously reported lower incidence among African-American and Latino populations in the USA, the incidence among these groups in the USA seems currently not very different from children of European origin in the USA [6,41]. The incidence rates of T1DM in most South American studies with standardized registries seems to be much lower than that among Latino people in the USA and lower than that among Spaniards living in Spain [40].
The incidence of T1DM among children of immigrant parents to Germany, Sweden and mainland Italy has been shown to correlate with the incidence in the country or region of origin of their parents, whether the incidence in the country of origin is higher or lower [43–45]; however, the incidence among immigrants from Pakistan to the UK (or their children) is similar to that among the native Britons [46,47], despite the very much lower incidence recorded in Karachi, Pakistan [6].
In summary, although there are clear ethnic differences in incidence rate of T1DM and strong evidence for a role of genetic factors, some of the abovementioned studies also support a role for yet unidentified environmental factors.
Seasonal variation in diagnosis of T1DM
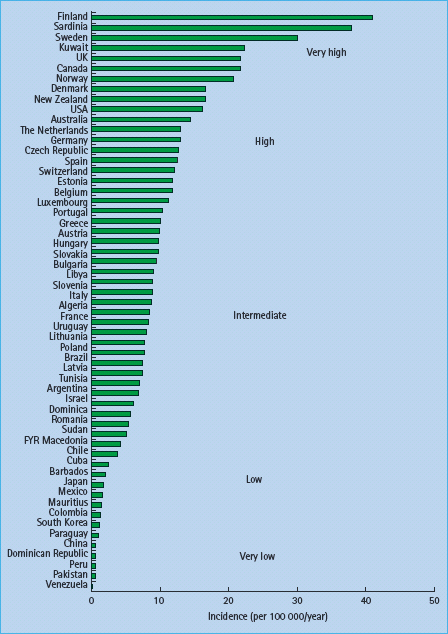
Several studies have reported a peak in the number of cases diagnosed in the autumn and winter, and a smaller proportion of the cases diagnosed in the spring or summer, consistent both in the northern and southern hemispheres [7,12]. Although reasonably consistent, there is some variation in exact peak and nadir between countries, age groups, sexes and periods. Generally, the degree of seasonal variation is stronger among those diagnosed at age 10–14 years than in younger children (Figure 3.3) [7]. Different methods have been used in the analysis of seasonal variations, and often with data covering relatively short periods of time and limited number of cases; the results are therefore not necessarily comparable. Interpretation of seasonal variation must be carried out in light of the long and variable preclinical period in T1DM, and it is speculated that viral or other periodic factors have a role in the timing of the precipitation or onset of the disease in susceptible individuals who would develop it sooner or later.
Figure 3.3 Seasonal variation in diagnosis of type 1 diabetes among > 22 000 children diagnosed 1989–1998 in European centers, by age at onset. * Age (years) at fi rst unsulin injection. Reproduced from Green & Patterson [7], with permission from Springer-Verlag.
Trends in incidence over time
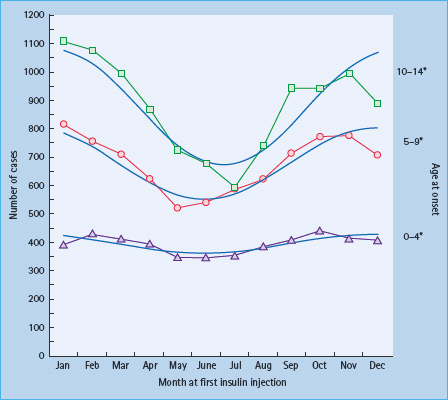
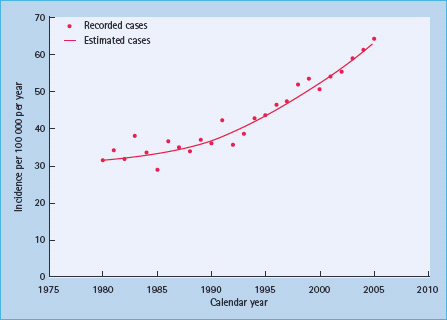
The methodology for population-based incidence registries was not standardized until the late 1980s, but a number of older data sources have been reviewed to assess possible time trends. There is a general impression that the incidence of T1DM increased strongly after the middle of the 20th century [48,49], although in Denmark, the incidence among 0- to 29-year-olds seemed stable from 1924 to the 1970s [11]. An analysis of incidence trends of more standardized registry data from 1960 to 1996 in 37 populations worldwide showed a significant increase in incidence rate over time in the majority of populations, with a steeper relative increase in low-incidence than in high-incidence populations [50]. In Finland, the incidence rate has increased linearly from the mid 1960s to the mid 1990s [51] and thereafter even more steeply, reaching almost 60 per 100 000/year in 2006 (Figure 3.4) [10].
Figure 3.4 Time trend in incidence rate of type 1 diabetes diagnosed before 15 years of age in Finland. Reproduced from Harjutsalo et al. [10], with permission from Elsevier.
In the 103 centers participating in the WHO DIAMOND project for at least 3 years during 1990–1999, the overall relative increase in incidence rate was 2.8% per year [6]. By continent, overall increasing trends per year were estimated at 5.3% in North America, 3.2% in Europe and 4.0% in Asia [6]. The only region with an overall decreasing trend was Central America and the West Indies [6].
In general, the increasing trend appeared to be strongest in the centers with high and very high incidence rates during the 1990s. In the centers with low and very low incidence rates, there were no significant increases in incidence rates over time. However, the 15-year (1989–2003) time trends in Europe reported to EURODIAB indicated an overall mean increase in incidence rate of 3.9% per year, with a tendency towards stronger relative increases in the countries with lowest average incidence rates during the first 5 years (1989–1994) [8].
Overall, the relative increase over time during the 1990s among the DIAMOND centers was most pronounced in the younger age group: 4.0% among 0- to 4-year-olds, 3.0% in the 5-to 9-year-olds and 2.1% in the 10- to 14-year-olds, similar for boys and girls in most centers. The pattern of a steeper relative increase among the youngest was seen in Europe and Oceania, but not in Asia and North America [6]. A smaller relative increase in incidence rate over time among older individuals than among younger ones has also been seen in European studies covering wider age ranges [14,17,18,52,53]. This latter observation is in line with a model where a certain pool of genetically susceptible individuals contract the disease at younger ages [14,17,54]. The few available data, however, are not entirely consistent with this idea. An increasing incidence rate has been seen also among older age groups in some populations [18,22,31], but the lack of standardized incidence data covering the whole age range makes it difficult to draw firm conclusions. Note that despite the time trends indicated in Sweden with decreasing average age of onset, the peak incidence rate remained around the age of puberty [14].
Familial clustering and twin studies
Besides providing clues regarding the relative importance of genetic and non-genetic factors in the etiology of disease, data on risk of T1DM among people with affected relatives may also aid the clinician in counseling of family members of newly diagnosed patients. Around 80–90% of newly diagnosed T1DM patients do not have any affected siblings or parents, but first-degree relatives of a person with T1DM are at increased risk. By the age of 20 years, approximately 4–6% of siblings of T1DM probands have been reported to develop T1DM in European origin populations [55–57], compared with around 0.2–1.0% in the corresponding background populations. The offspring of affected fathers have 1.5–3 times increased risk of T1DM compared with the offspring of affected mothers [58,59]. By 20 years of age, 5–8% of the offspring of men with diabetes, but only 2–5% of the offspring of women with diabetes have been found to be affected. There is currently no accepted explanation for this phenomenon.
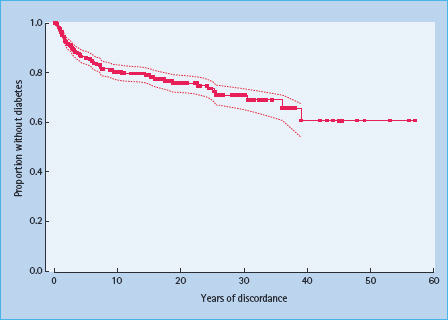
Given the well-established effect of genetic factors, there is no surprise that the concordance rate for T1DM in monozygotic (MZ) twins is much higher than that in dizygotic (DZ) twins [60,61]. In one study from North America, the estimated cumulative risk 10 years after onset in the proband (diagnosed before age 40 years) was about 25% in MZ twins and about 11% in DZ twins [62]. Corresponding 10-year cumulative risks from Finland were estimated to 32% for MZ twins and 3.2% for DZ twins [61]. Comparable estimates were also found in the Danish twin registry, although the exact age at onset was not known in this study [60]. In general, the risk for the co-twins was higher and the discordance time shorter the earlier the onset in the proband [61–63]. In a long-term follow-up of discordant MZ twins from the USA and the UK, more than 50% of MZ pairs remained discordant for T1DM (Figure 3.5) [63]. Because MZ twins share 100% of their genomic DNA, this suggests that genetic susceptibility is in most cases not sufficient for the development of disease.
Figure 3.5 Diabetes-free survival in non-diabetic monozygotic twins whose co-twin had type 1 diabetes, after several years of follow-up. Dotted lines represent 95% confidence intervals. Reproduced from Redondo et al. [63], with permission from Springer-Verlag.
Despite the relatively high proportion of MZ twins being discordant for clinical T1DM, many of the non-diabetic co-.wins of patients with T1DM develop islet autoimmunity [64]. Together with the limited variation in prevalence of positivity for islet autoantibodies between countries [54] and animal studies suggesting a two-stage disease process [65], this supports the idea that environmental factors may have a more important role in the progression from islet autoimmunity to overt disease.
Environmental risk factors for T1DM: clues from epidemiologic studies
The time trends described above must be ascribed to some change in the environment, even in the event that the increase is brought about by a change in the age distribution. Some of the variation in incidence rate between European countries can be explained by differences in the frequency of HLA susceptibility genotypes [66], but clearly not all [67]. It has recently been suggested that the proportion of newly diagnosed patients with T1DM who carry the highest risk genotype (DR3-DQ2/DR4-DQ8) has decreased over time (reviewed in [68]). It may be speculated that increased exposure to some risk factor or decreased exposure to some protective factor have caused more individuals with moderate risk (“permissive”) genotypes (e.g. either DR3-DQ2 or DR4-DQ8, but not both) to develop T1DM in recent years. Although there is overwhelming evidence for an essential role of genetic factors in the etiology of T1DM, available evidence strongly suggests that one or more non-genetic factors are also involved; however, the nature of these putative factors are not well known.
In general, environmental factors may be envisioned to have a role in the following:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree